Warm Up List the first second and third






- Slides: 13

Warm Up • List the first, second, and third steps of the scientific method: 1. 2. 3.

Measurement Chapter 1. 3

Standards of Measurement • A standard is an exact quantity that people agree to use to compare measurements • Measurements must include a number and a unit Measurements are either English or SI (International System of Units)

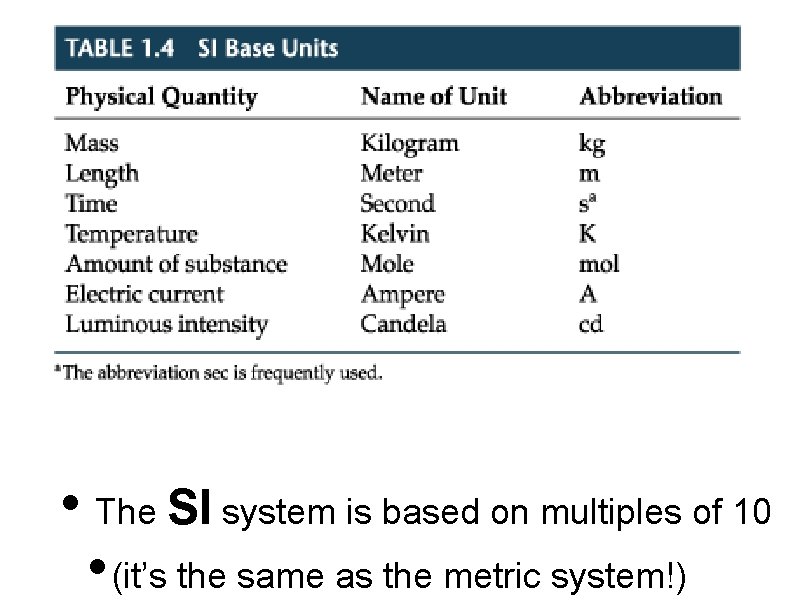
• The SI system is based on multiples of 10 • (it’s the same as the metric system!)

Distance • Length- is the distance between two points Prefix Symbol Factor # Factor Word Kilo k 1000 Thousand Hecto h 100 Hundred Deca da 10 Ten Base --- 1 M, L, g Deci d 0. 1 Tenth Centi c 0. 01 Hundredth Milli m 0. 001 Thousandth

Distance Conversions • How many millimeters are in 2. 40 m? • How many meters are equal to 742 cm?

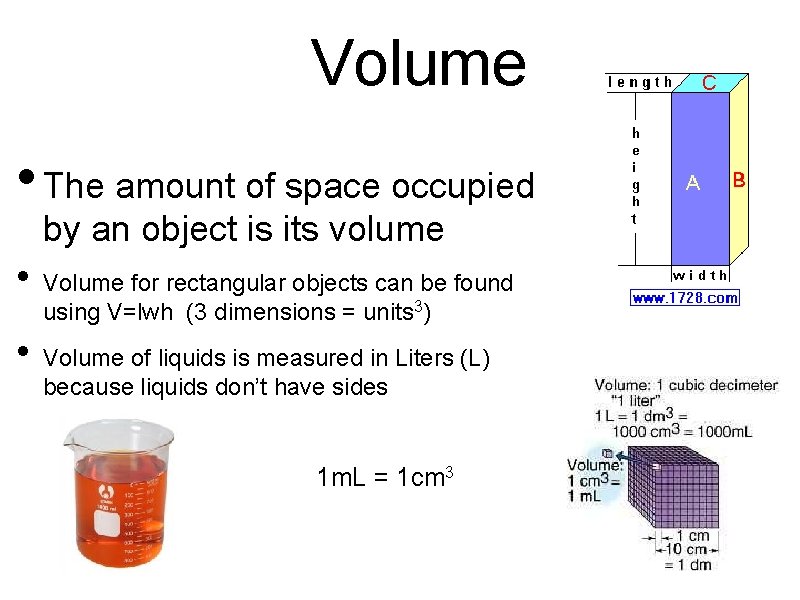
Volume • The amount of space occupied by an object is its volume • Volume for rectangular objects can be found using V=lwh (3 dimensions = units 3) • Volume of liquids is measured in Liters (L) because liquids don’t have sides • 1 m. L = 1 cm 3

Mass Just because an object is large, it doesn’t have to have a lot of matter – think of a balloon • Mass is a measurement of the quantity of matter in an object

Mass versus Weight • Mass is the amount of matter an object contains. • Doesn’t change from the Earth to the Moon. • Weight is the force of gravity on a mass. • Much less on the Moon than the Earth.

• Density is the mass Density per unit of volume of a material. • Units are g/m. L or g/cm 3 • Different materials have different densities. • 25 grams of water takes up 25 m. L of space, so the density of water can be found using g/m. L 25 g/25 m. L = 1 g/1 m. L = 1 g/m. L

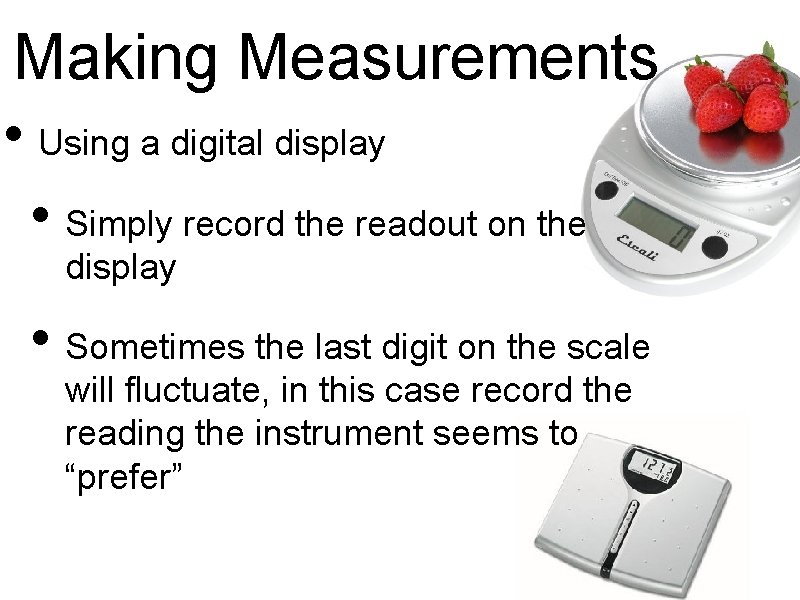
Making Measurements • Using a digital display • Simply record the readout on the display • Sometimes the last digit on the scale will fluctuate, in this case record the reading the instrument seems to “prefer”

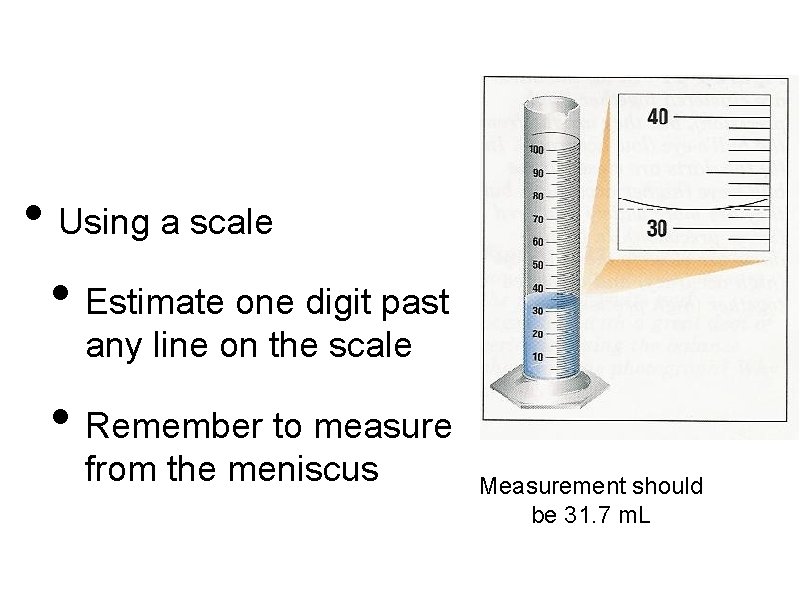
• Using a scale • Estimate one digit past any line on the scale • Remember to measure from the meniscus Measurement should be 31. 7 m. L

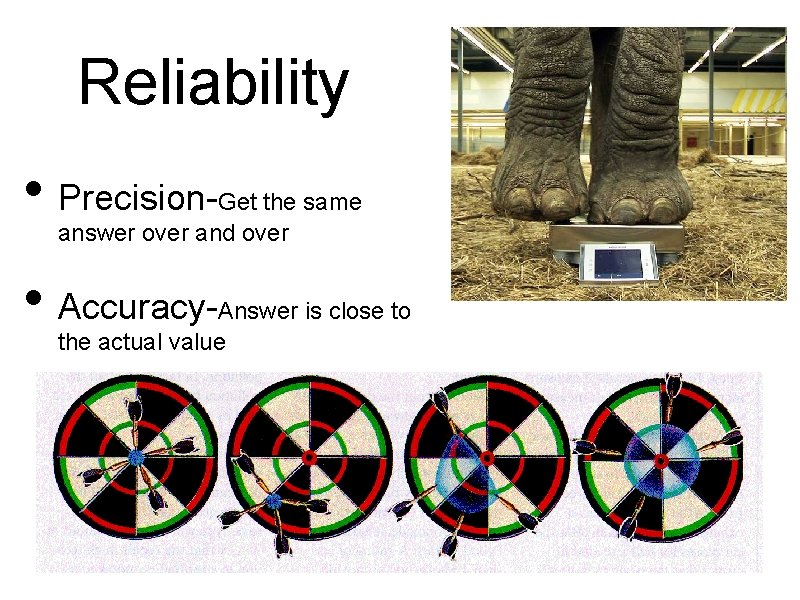
Reliability • Precision- Get the same answer over and over • Accuracy- Answer is close to the actual value