Warm up Have HW out for a stamp






- Slides: 17

Warm up (Have HW out for a stamp) • Magnesium and hydrochloric acid react to form magnesium chloride and hydrogen gas. • Mg(s) + HCl Mg. Cl 2 (aq) + H 2 (g) 1. Balance the equation 2. How many grams of magnesium chloride can form if 3. 75 grams of magnesium reacts with plenty of HCl?

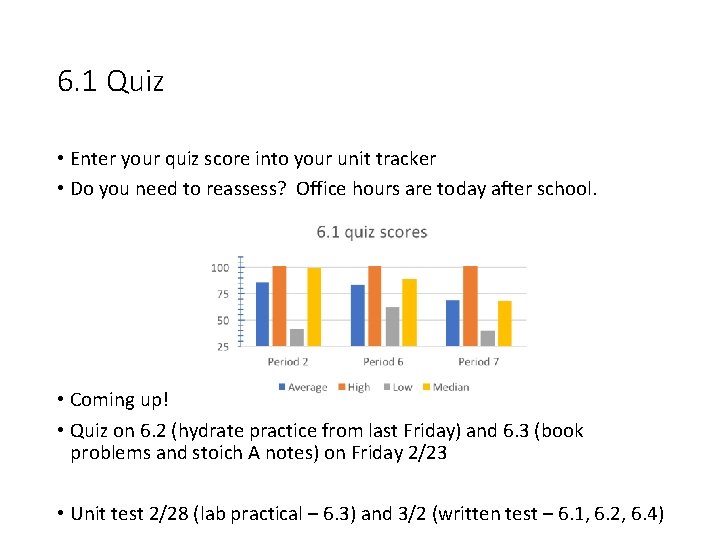
6. 1 Quiz • Enter your quiz score into your unit tracker • Do you need to reassess? Office hours are today after school. • Coming up! • Quiz on 6. 2 (hydrate practice from last Friday) and 6. 3 (book problems and stoich A notes) on Friday 2/23 • Unit test 2/28 (lab practical – 6. 3) and 3/2 (written test – 6. 1, 6. 2, 6. 4)

HW – questions?

Limiting and excess reactants • Consider the balanced reaction for s’mores. 2 Gc + 1 M + 2 Cp Gc 2 MCp 2 • If you have 6 graham crackers, 6 marshmallows and 6 chocolate pieces… • Which ingredients will get all used up and limit the number of s’mores you can make? • Which ingredients will be in excess?

‘Limiting’ and ‘excess’ reactants • Limiting reactant: the reactant that limits the amounts of the other reactants that can combine and limits the amount of product that can form in a chemical reaction. • Excess reactant: the substance that is not used up completely in a reaction.

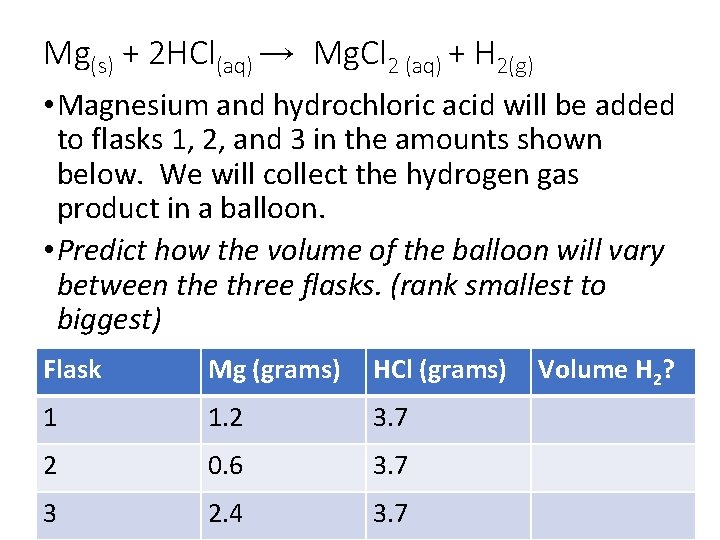
Mg(s) + 2 HCl(aq) → Mg. Cl 2 (aq) + H 2(g) • Magnesium and hydrochloric acid will be added to flasks 1, 2, and 3 in the amounts shown below. We will collect the hydrogen gas product in a balloon. • Predict how the volume of the balloon will vary between the three flasks. (rank smallest to biggest) Flask Mg (grams) HCl (grams) 1 1. 2 3. 7 2 0. 6 3. 7 3 2. 4 3. 7 Volume H 2?

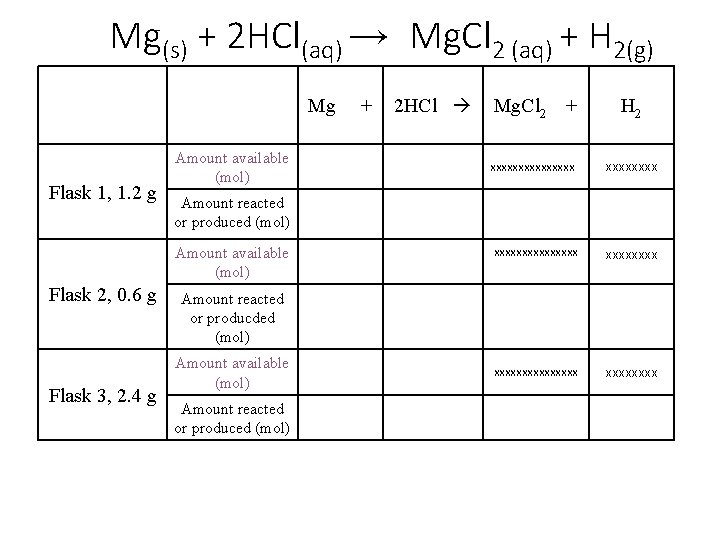
Mg(s) + 2 HCl(aq) → Mg. Cl 2 (aq) + H 2(g) Mg Flask 1, 1. 2 g Amount available (mol) Flask 3, 2. 4 g 2 HCl Mg. Cl 2 + H 2 xxxxxxxxxxxxxxx xxxxxxxx Amount reacted or produced (mol) Amount available (mol) Flask 2, 0. 6 g + Amount reacted or producded (mol) Amount available (mol) Amount reacted or produced (mol)

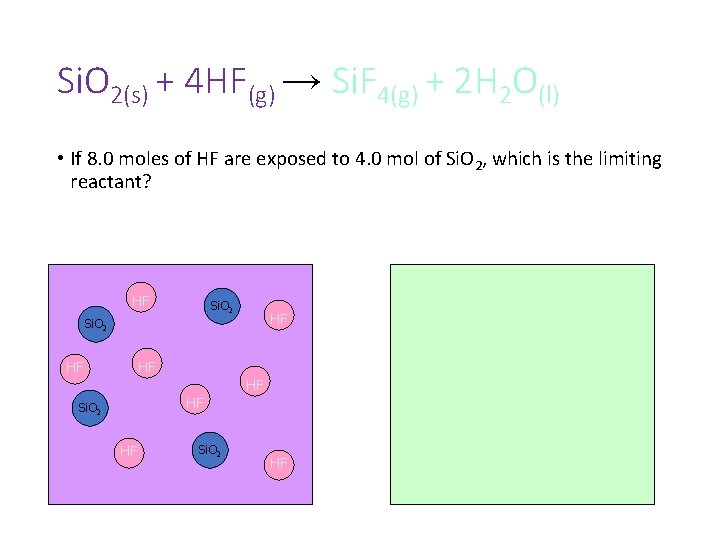
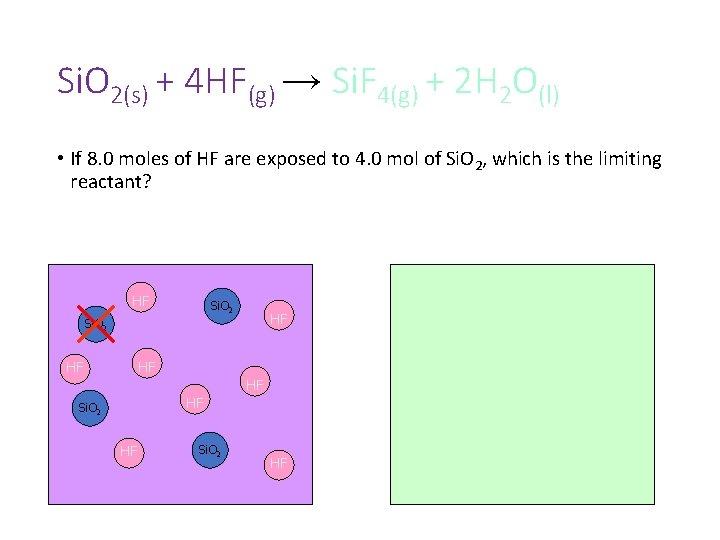
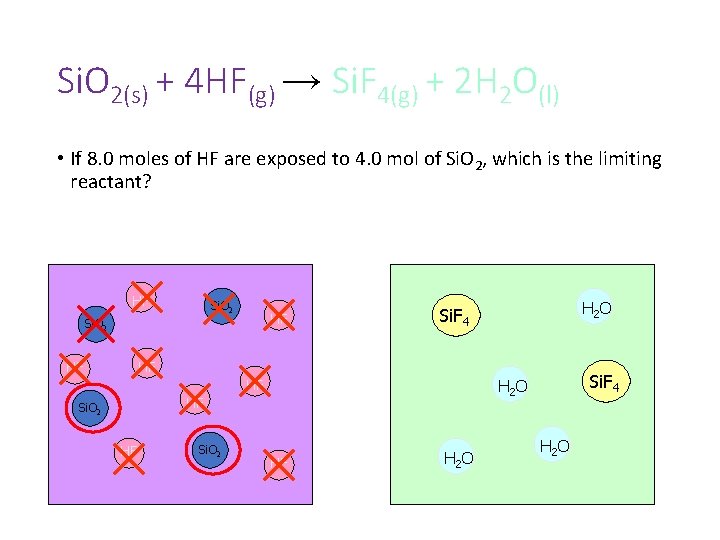
Si. O 2(s) + 4 HF(g) → Si. F 4(g) + 2 H 2 O(l) • If 8. 0 moles of HF are exposed to 4. 0 mol of Si. O 2, which is the limiting reactant?

Si. O 2(s) + 4 HF(g) → Si. F 4(g) + 2 H 2 O(l) • If 8. 0 moles of HF are exposed to 4. 0 mol of Si. O 2, which is the limiting reactant? HF Si. O 2 HF

Si. O 2(s) + 4 HF(g) → Si. F 4(g) + 2 H 2 O(l) • If 8. 0 moles of HF are exposed to 4. 0 mol of Si. O 2, which is the limiting reactant? HF Si. O 2 HF

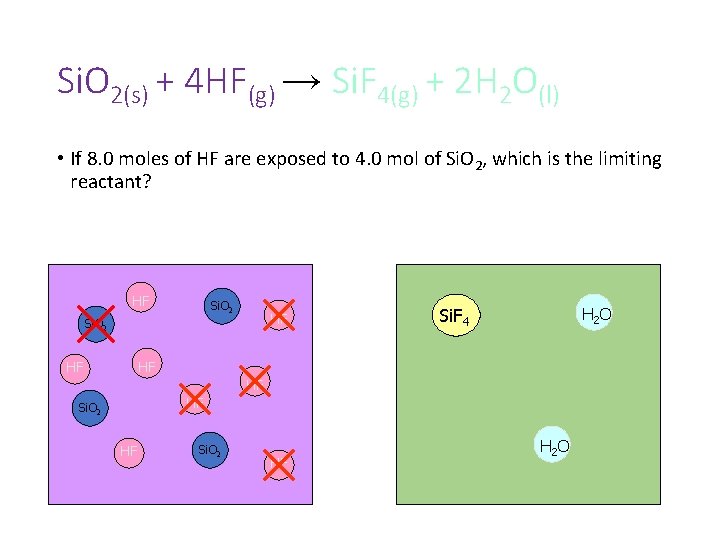
Si. O 2(s) + 4 HF(g) → Si. F 4(g) + 2 H 2 O(l) • If 8. 0 moles of HF are exposed to 4. 0 mol of Si. O 2, which is the limiting reactant? HF Si. O 2 HF Si. F 4 H 2 O HF HF HF Si. O 2 HF H 2 O

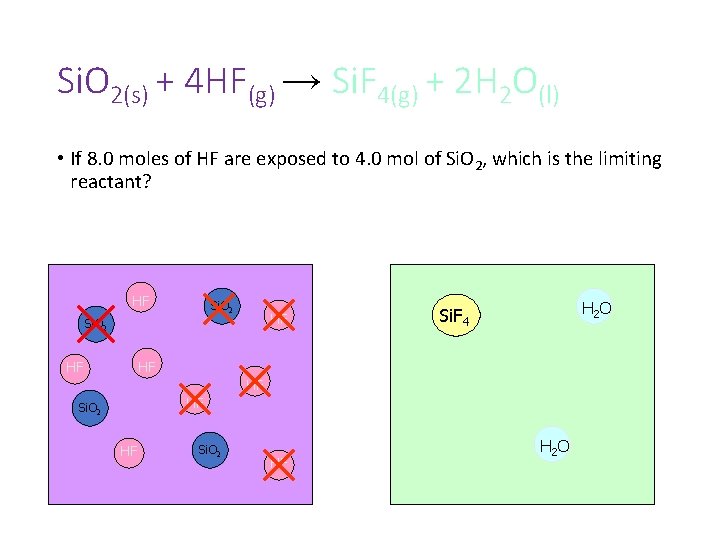
Si. O 2(s) + 4 HF(g) → Si. F 4(g) + 2 H 2 O(l) • If 8. 0 moles of HF are exposed to 4. 0 mol of Si. O 2, which is the limiting reactant? HF Si. O 2 HF H 2 O Si. F 4 HF HF HF Si. O 2 HF H 2 O

Si. O 2(s) + 4 HF(g) → Si. F 4(g) + 2 H 2 O(l) • If 8. 0 moles of HF are exposed to 4. 0 mol of Si. O 2, which is the limiting reactant? HF Si. O 2 HF H 2 O Si. F 4 HF Si. O 2 Si. F 4 H 2 O HF H 2 O

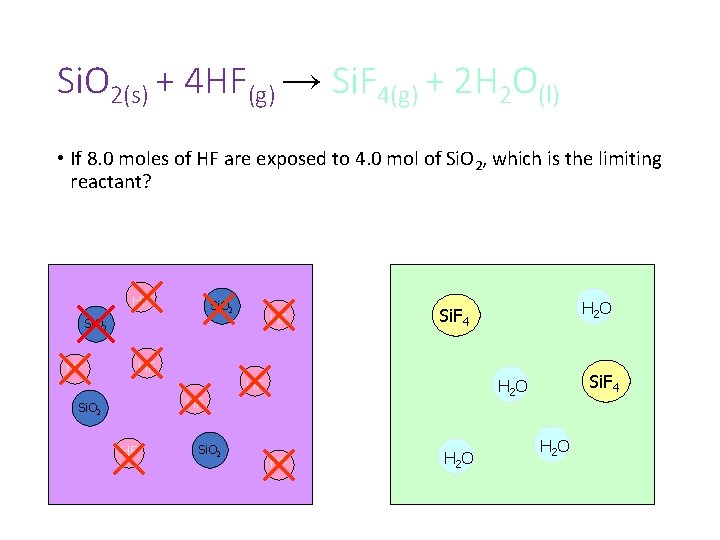
Si. O 2(s) + 4 HF(g) → Si. F 4(g) + 2 H 2 O(l) • If 8. 0 moles of HF are exposed to 4. 0 mol of Si. O 2, which is the limiting reactant? HF Si. O 2 HF H 2 O Si. F 4 HF HF Si. O 2 Si. F 4 H 2 O HF HF H 2 O

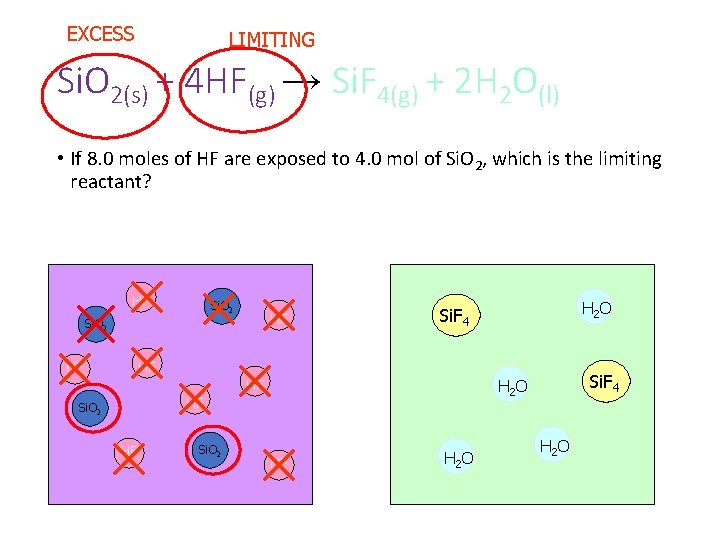
EXCESS LIMITING Si. O 2(s) + 4 HF(g) → Si. F 4(g) + 2 H 2 O(l) • If 8. 0 moles of HF are exposed to 4. 0 mol of Si. O 2, which is the limiting reactant? HF Si. O 2 HF H 2 O Si. F 4 HF HF Si. O 2 Si. F 4 H 2 O HF HF H 2 O

For most chemical reactions, one reactant is consumed before the other and it limits the amount of product that can be formed. How to recognize and SOLVE these types of problems: If quantities are given for more than one reactant, you must treat it as a limiting reactant situation: 1. Using reactant “A” calculate how many moles of product can be formed. This assumes that “B” is in excess. 2. Using reactant “B” calculate how many moles of product can be formed. This assumes that “A” is in excess. 3. The reactant that forms the least amount of product is the limiting reactant. 4. Calculate grams of product formed based on limiting reactant.

Closure • Let’s revisit our warm up question… • Magnesium and hydrochloric acid react to form magnesium chloride and hydrogen gas. • Mg(s) + 2 HCl Mg. Cl 2 (aq) + H 2 (g) • How many grams of magnesium chloride can form if 3. 75 grams of magnesium reacts with 8. 31 g of HCl? • PRACTICE!! Homework: LR WS