Warm Up Draw the dot diagrams for H


- Slides: 12

Warm Up Draw the dot diagrams for: H

Lewis Structures

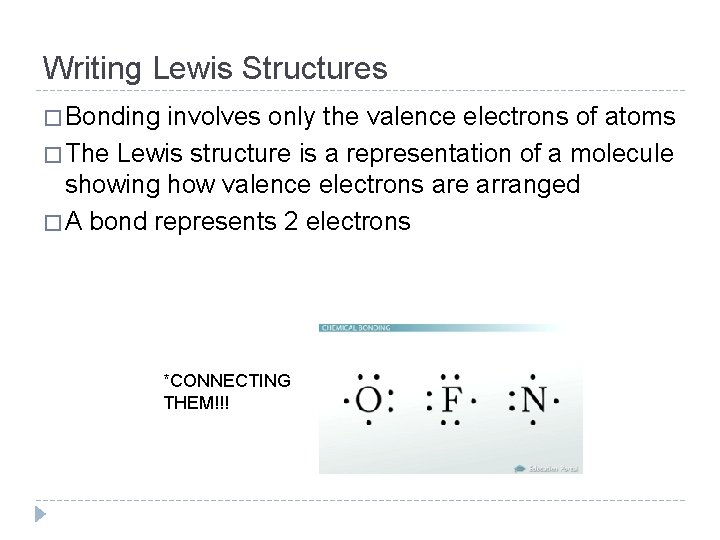
Writing Lewis Structures � Bonding involves only the valence electrons of atoms � The Lewis structure is a representation of a molecule showing how valence electrons are arranged � A bond represents 2 electrons *CONNECTING THEM!!!

Lewis Structure Vocabulary -Duet Rule – want 2 electrons in outer shell to be stable -Octet Rule – want 8 electrons in outer shell to be stable -Bonding Pair – pair of electrons shared between two atoms -Lone Pairs – electrons not involved in bonding

Steps to Drawing Lewis Structures 1. 2. 3. Find the total number of valence electrons from all atoms involved Use one pair of electrons to form a bond between each pair of bound atoms (use line) Arrange the remaining electrons to satisfy the duet or octet rule *Note the valence electrons are shared between all the atoms. Ex: So the valence electrons for an Oxygen atom are not just stuck to that Oxygen.

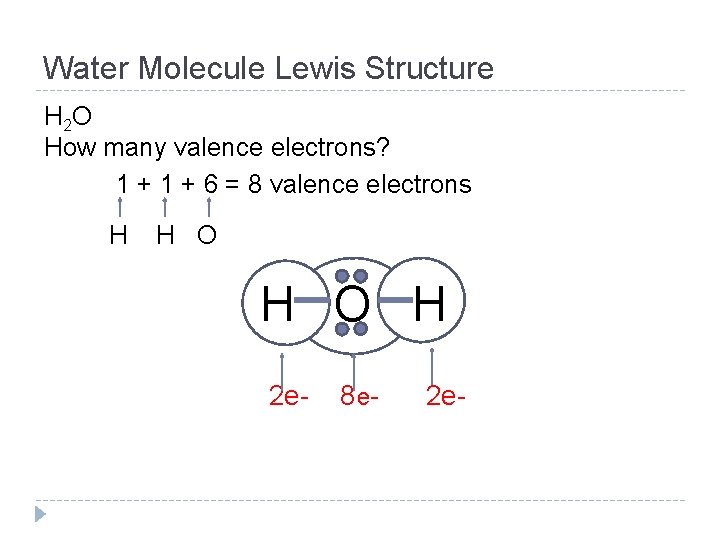
Water Molecule Lewis Structure H 2 O How many valence electrons? 1 + 6 = 8 valence electrons H H O H 2 e- 8 e- 2 e-

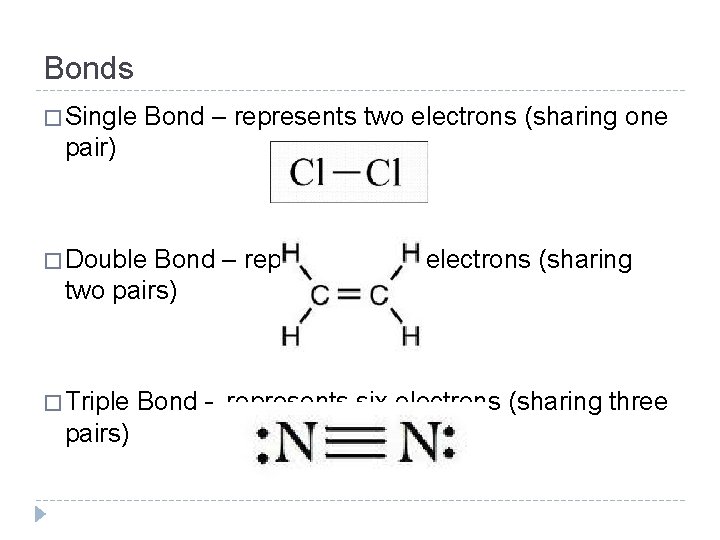
Bonds � Single Bond – represents two electrons (sharing one pair) � Double Bond – represents four electrons (sharing two pairs) � Triple pairs) Bond – represents six electrons (sharing three

When to USE double or triple bonds �Use a double or triple bond, if the electrons around element are SHORT of the octet rule (does not have 8 electrons around it!) �Gets more electrons around it!

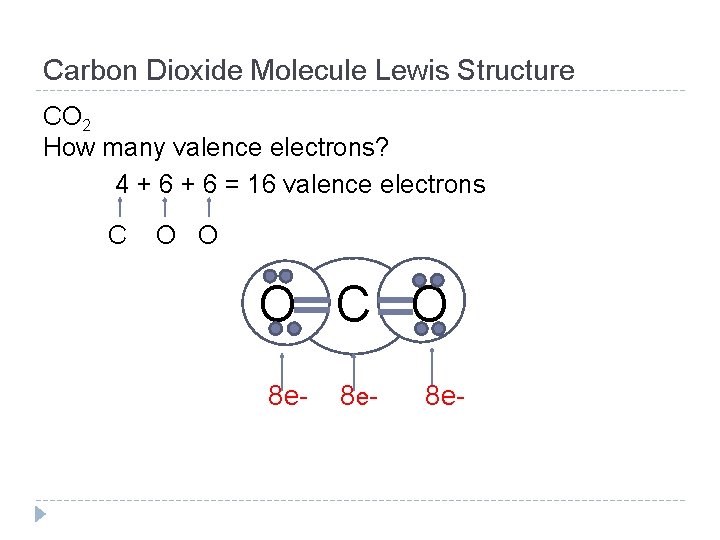
Carbon Dioxide Molecule Lewis Structure CO 2 How many valence electrons? 4 + 6 = 16 valence electrons C O O O C O 8 e- 8 e-

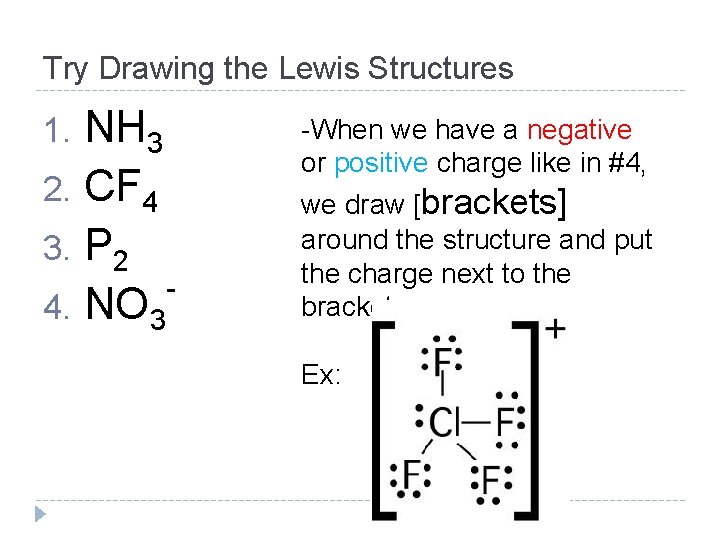
Try Drawing the Lewis Structures NH 3 2. CF 4 3. P 2 4. NO 3 1. -When we have a negative or positive charge like in #4, we draw [brackets] around the structure and put the charge next to the bracket Ex:

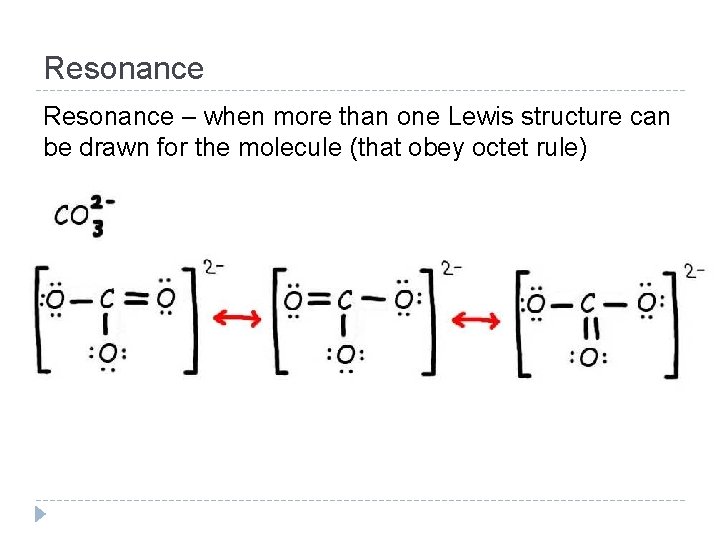
Resonance – when more than one Lewis structure can be drawn for the molecule (that obey octet rule)

Exit Slip Draw the lewis structure for H 2 S