WARFARIN AN OVERVIEW HEMOSTASIS VASCULAR SPASM PLATELET PLUG





![INTERNATIONAL NORMALISED RATIO (INR) INR = [PTpt] ISI [PTRef] PTpt – prothrombin time of INTERNATIONAL NORMALISED RATIO (INR) INR = [PTpt] ISI [PTRef] PTpt – prothrombin time of](https://slidetodoc.com/presentation_image_h2/07198049b822acbb6006f426629485c5/image-14.jpg)







- Slides: 27

WARFARIN AN OVERVIEW

HEMOSTASIS Ø VASCULAR SPASM Ø PLATELET PLUG Ø BLOOD COAGULATION Ø GROWTH OF FIBROUS TISSUE IN CLOT

WHEN DOES BLOOD COAGULATE? Procoagulants > Anticoagulants n Injury to blood vessel n Blood stasis n

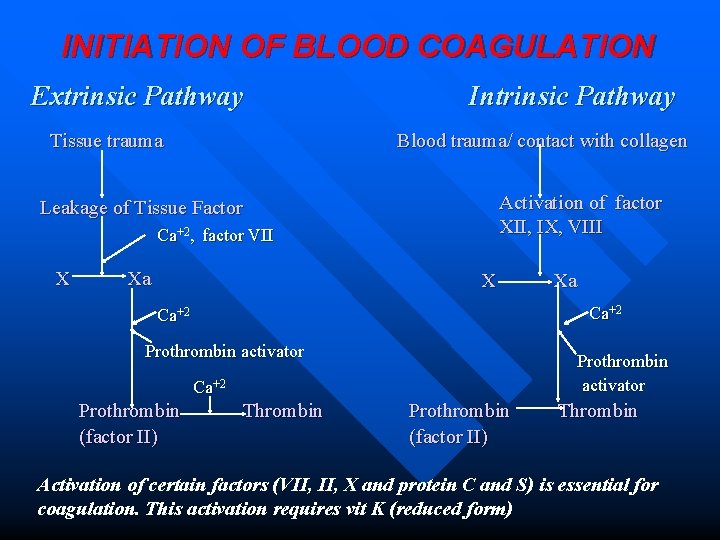
INITIATION OF BLOOD COAGULATION Extrinsic Pathway Tissue trauma Intrinsic Pathway Blood trauma/ contact with collagen Activation of factor XII, IX, VIII Leakage of Tissue Factor Ca+2, factor VII X Xa X Ca+2 Prothrombin activator Ca+2 Prothrombin (factor II) Xa Thrombin Prothrombin (factor II) Thrombin Activation of certain factors (VII, X and protein C and S) is essential for coagulation. This activation requires vit K (reduced form)

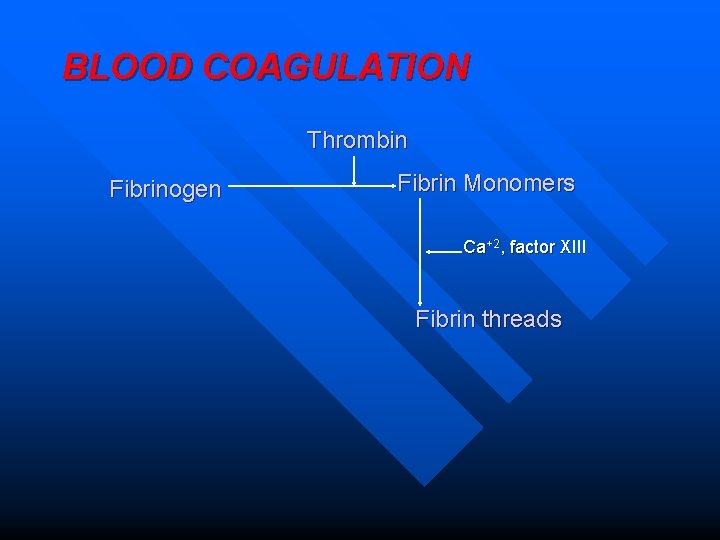
BLOOD COAGULATION Thrombin Fibrinogen Fibrin Monomers Ca+2, factor XIII Fibrin threads

ANTICOAGULANTS Three classes Ø Heparin and Low Molecular Weight Heparins (e. g. enoxaparin, dalteparin) Ø Coumarin Derivatves e. g. Warfarin, Acenocoumarol Ø Indandione Derivatves e. g. Phenindione, Anisindione

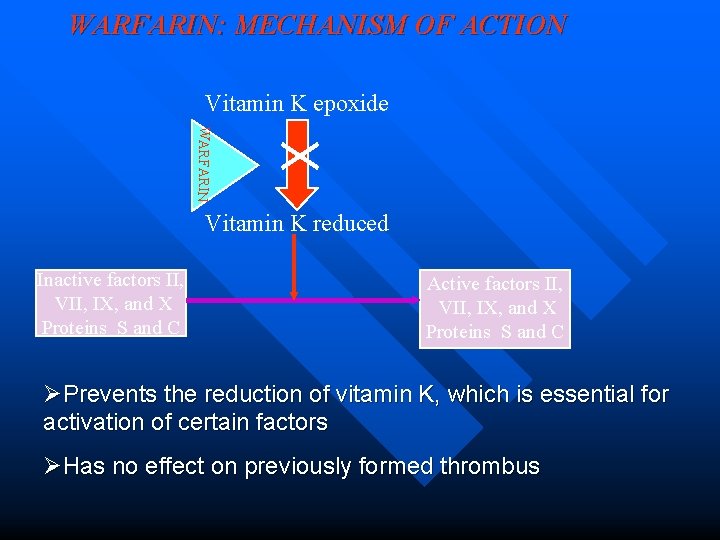
WARFARIN: MECHANISM OF ACTION Vitamin K epoxide WARFARIN Vitamin K reduced Inactive factors II, VII, IX, and X Proteins S and C Active factors II, VII, IX, and X Proteins S and C ØPrevents the reduction of vitamin K, which is essential for activation of certain factors ØHas no effect on previously formed thrombus

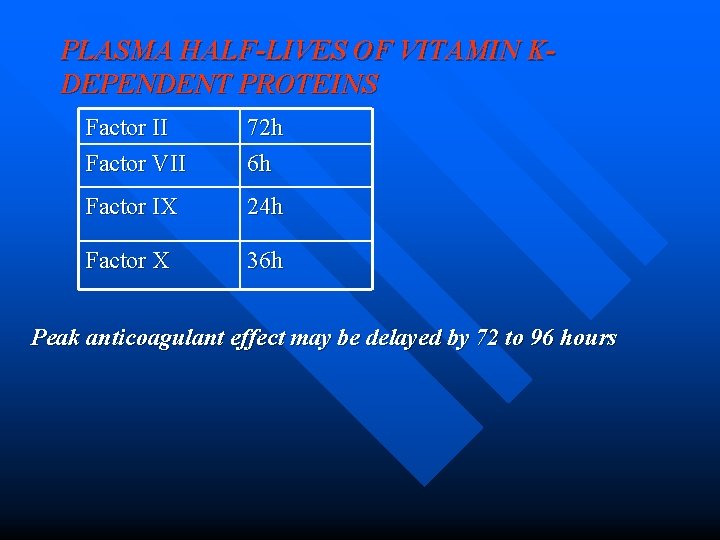
PLASMA HALF-LIVES OF VITAMIN KDEPENDENT PROTEINS Factor II Factor VII 72 h 6 h Factor IX 24 h Factor X 36 h Peak anticoagulant effect may be delayed by 72 to 96 hours

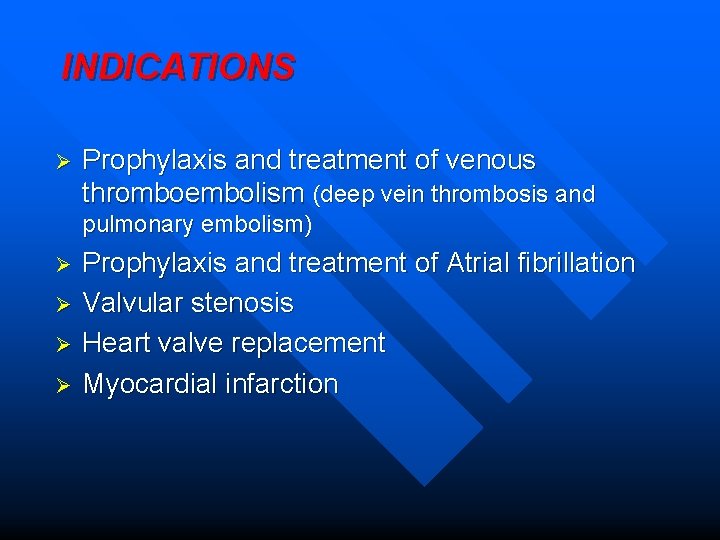
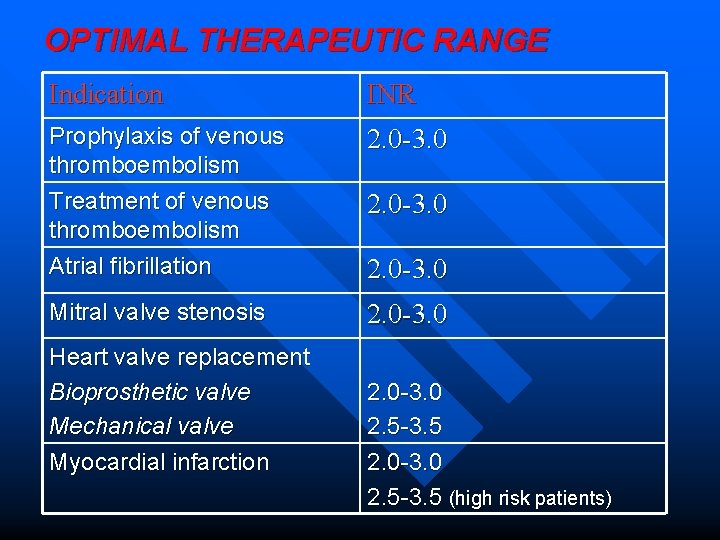
INDICATIONS Ø Prophylaxis and treatment of venous thromboembolism (deep vein thrombosis and pulmonary embolism) Ø Ø Prophylaxis and treatment of Atrial fibrillation Valvular stenosis Heart valve replacement Myocardial infarction

WHY TO MONITOR WARFARIN THERAPY? Narrow therapeutic range n Can increase risk of bleeding n

MONITORING OF WARFARIN THERAPY Ø Ø Ø Prothrombin time PT ratio INR (International Normalized Ratio)

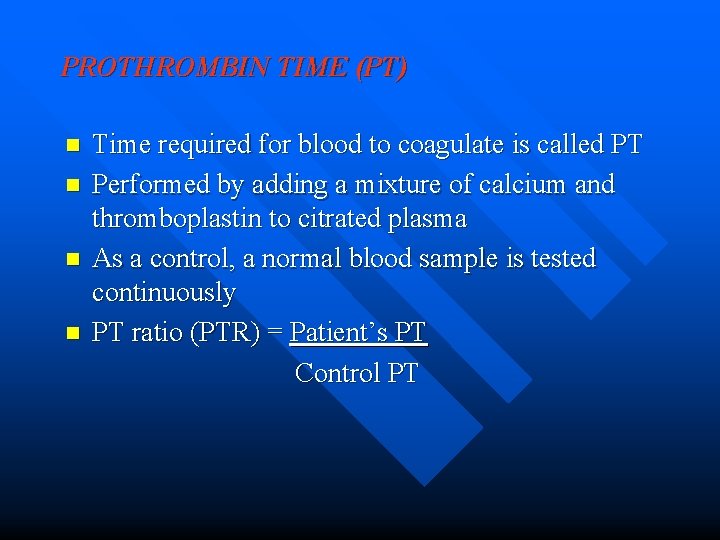
PROTHROMBIN TIME (PT) n n Time required for blood to coagulate is called PT Performed by adding a mixture of calcium and thromboplastin to citrated plasma As a control, a normal blood sample is tested continuously PT ratio (PTR) = Patient’s PT Control PT

PROBLEMS WITH PT/PTR Thromboplastins are extracts from brain, lung or placenta of animals n Thromboplastins from various manufacturers differ in their sensitivity to prolong PT n May result in erratic control of anticoagulant therapy n
![INTERNATIONAL NORMALISED RATIO INR INR PTpt ISI PTRef PTpt prothrombin time of INTERNATIONAL NORMALISED RATIO (INR) INR = [PTpt] ISI [PTRef] PTpt – prothrombin time of](https://slidetodoc.com/presentation_image_h2/07198049b822acbb6006f426629485c5/image-14.jpg)
INTERNATIONAL NORMALISED RATIO (INR) INR = [PTpt] ISI [PTRef] PTpt – prothrombin time of patient PTRef – prothrombin time of normal pooled sample ISI – International Sensitivity Index

OPTIMIZING WARFARIN THERAPY n n n Dosage to be individualized according to patient’s INR response. Use of large loading dose may lead to hemorrhage and other complications. Initial dose: 2 -5 mg once daily Maintenance dose: 2 -10 mg once daily Immediate anticoagulation required: Start heparin along with loading dose of warfarin 10 mg. Heparin is usually discontinued after 4 -5 days. Before discontinuing, ensure INR is in therapeutic range for 2 consecutive days n Monitor daily until INR is in therapeutic range, then 3 times weekly for 1 -2 weeks, then less often (every 4 to 6 weeks)

OPTIMAL THERAPEUTIC RANGE Indication INR Prophylaxis of venous thromboembolism Treatment of venous thromboembolism Atrial fibrillation 2. 0 -3. 0 Mitral valve stenosis 2. 0 -3. 0 Heart valve replacement Bioprosthetic valve Mechanical valve Myocardial infarction 2. 0 -3. 0 2. 5 -3. 5 (high risk patients)

FACTORS INFLUENCING DOSE RESPONSE Inaccurate lab testing n Poor patient compliance n Concomitant medications n Levels of dietary vitamin K n Alcohol n Hepatic dysfunction n Fever n

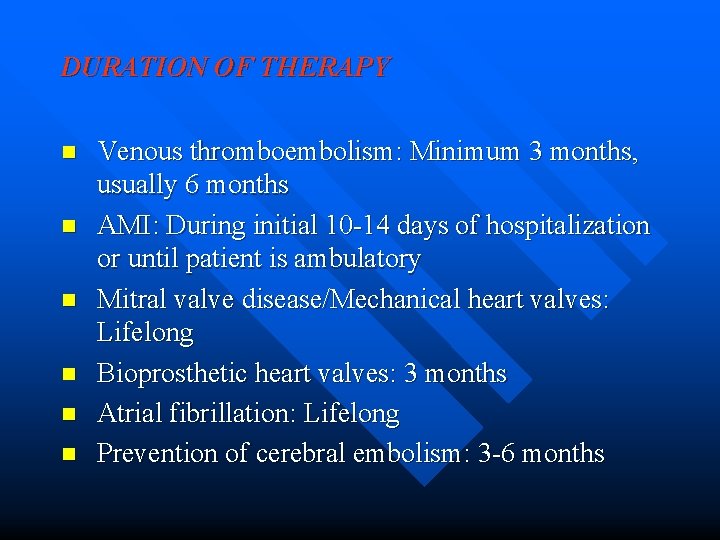
DURATION OF THERAPY n n n Venous thromboembolism: Minimum 3 months, usually 6 months AMI: During initial 10 -14 days of hospitalization or until patient is ambulatory Mitral valve disease/Mechanical heart valves: Lifelong Bioprosthetic heart valves: 3 months Atrial fibrillation: Lifelong Prevention of cerebral embolism: 3 -6 months

CONTARINDICATIONS AND PRECAUTIONS Ø Ø Ø Ø Hypersensitivity to warfarin Condition with risk of hemorrhage Hemorrhagic tendency Inadequate laboratory techniques Protein C & S deficiency Vitamin K deficiency Intramuscular injections

SIDE EFFECTS Hemorrhage Ø Skin necrosis Ø Purple toe syndrome Ø Microembolization Ø Teratogenecity Agranulocytosis, leukopenia, diarrhoea, nausea, anorexia. Ø

SWITCHOVER FROM ONE BRAND OF WARFARIN TO ANOTHER/ ACENOCOUMAROL Check patient’s INR n Start with dose of 2 mg; increase dose slowly as required n

COMPARISON WITH ACENOCOUMAROL

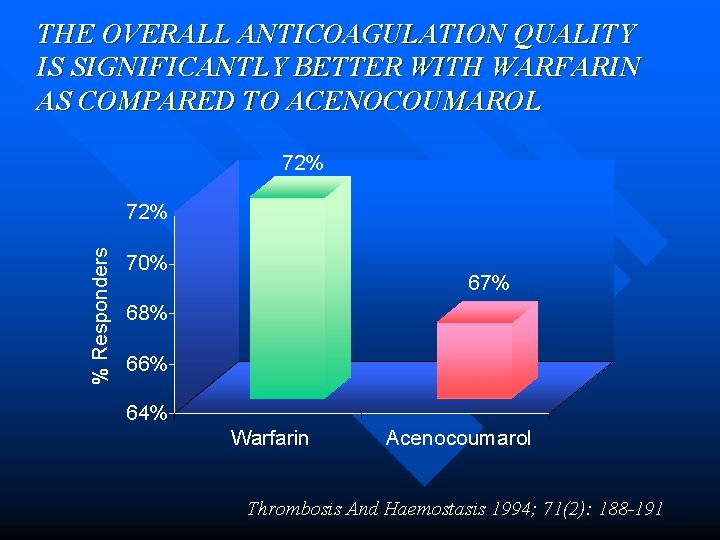
THE OVERALL ANTICOAGULATION QUALITY IS SIGNIFICANTLY BETTER WITH WARFARIN AS COMPARED TO ACENOCOUMAROL 72% % Responders 72% 70% 67% 68% 66% 64% Warfarin Acenocoumarol Thrombosis And Haemostasis 1994; 71(2): 188 -191

RECENT TRIALS ON WARFARIN

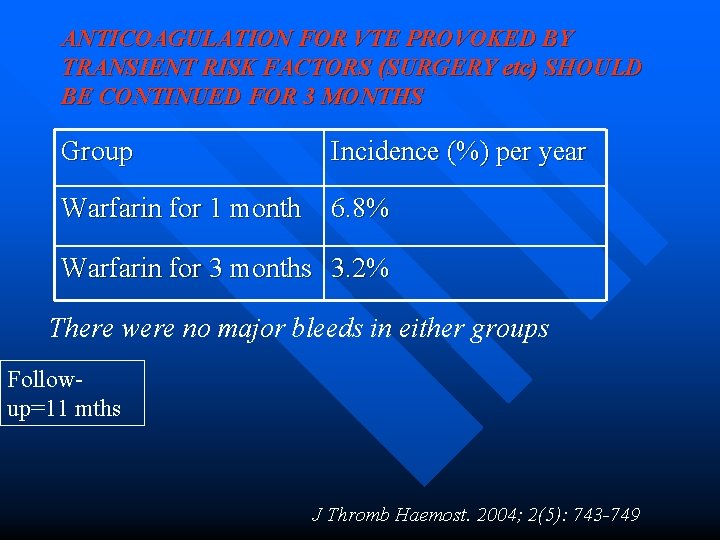
ANTICOAGULATION FOR VTE PROVOKED BY TRANSIENT RISK FACTORS (SURGERY etc) SHOULD BE CONTINUED FOR 3 MONTHS Group Incidence (%) per year Warfarin for 1 month 6. 8% Warfarin for 3 months 3. 2% There were no major bleeds in either groups Followup=11 mths J Thromb Haemost. 2004; 2(5): 743 -749

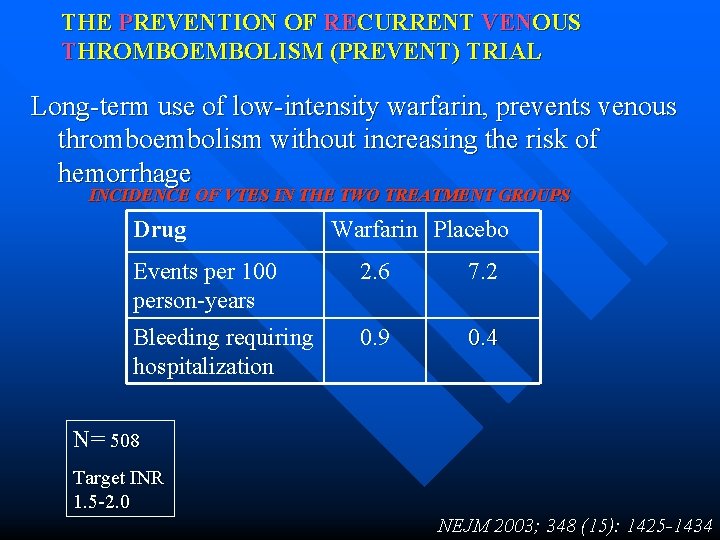
THE PREVENTION OF RECURRENT VENOUS THROMBOEMBOLISM (PREVENT) TRIAL Long-term use of low-intensity warfarin, prevents venous thromboembolism without increasing the risk of hemorrhage INCIDENCE OF VTES IN THE TWO TREATMENT GROUPS Drug Warfarin Placebo Events per 100 person-years 2. 6 7. 2 Bleeding requiring hospitalization 0. 9 0. 4 N= 508 Target INR 1. 5 -2. 0 NEJM 2003; 348 (15): 1425 -1434

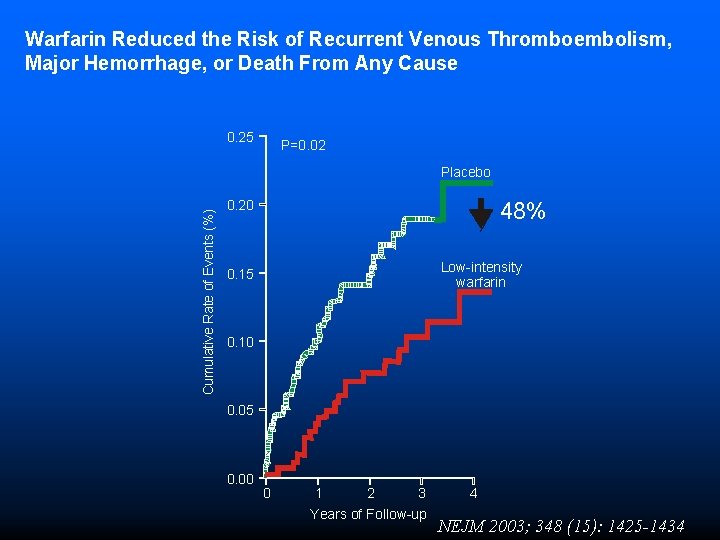
Warfarin Reduced the Risk of Recurrent Venous Thromboembolism, Major Hemorrhage, or Death From Any Cause 0. 25 P=0. 02 Cumulative Rate of Events (%) Placebo 0. 20 48% Low-intensity warfarin 0. 15 0. 10 0. 05 0. 00 0 1 2 3 Years of Follow-up 4 NEJM 2003; 348 (15): 1425 -1434