VSPER Shapes and Polarity of Molecules Adapted from
- Slides: 33

VSPER Shapes and Polarity of Molecules Adapted from: Pearson Education, Inc. Publishing as Benjamin Cummings 1

Shapes of Molecules n Molecules (covalent chemicals) form certain shapes depending on how many lone and bonding pairs of electrons it has. Bonding pair – these can also be drawn as straight lines Lone pair n Because the electron pairs repel each other we get certain shapes being formed. These are due to a certain rule called VSEPR (Valence Shell Electron Pair Repulsion)

VSEPR Theory n Based on Lewis structures we can know the shape or “geometry” of molecules n VSEPR, as the name suggests, predicts geometry based on the repulsion of electron pairs (bonding pairs and lone pairs) n Electrons around the central nucleus repel each other. Thus, resulting structures have atoms maximally spread out.

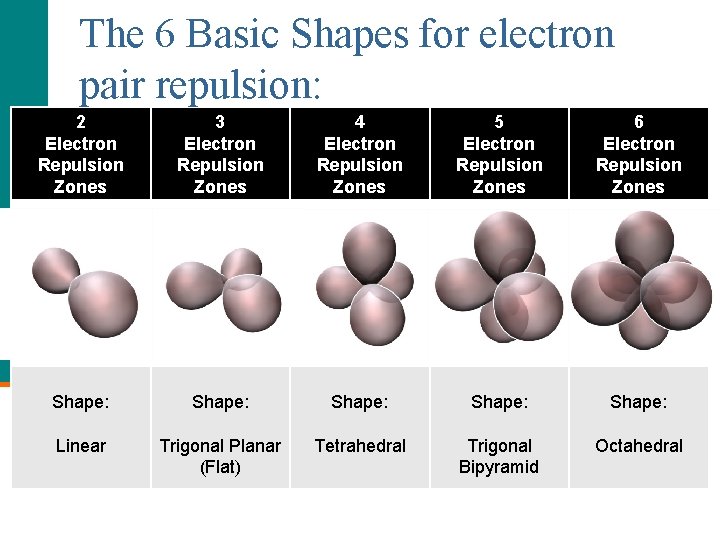
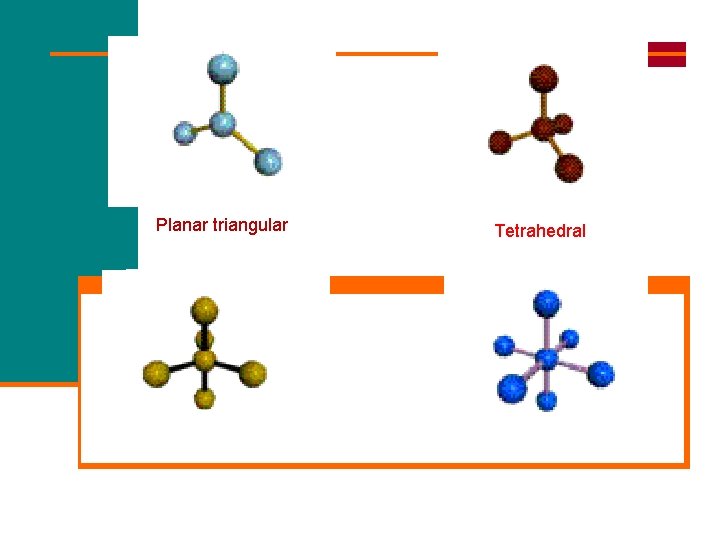
The 6 Basic Shapes for electron pair repulsion: 2 Electron Repulsion Zones 3 Electron Repulsion Zones 4 Electron Repulsion Zones 5 Electron Repulsion Zones 6 Electron Repulsion Zones Shape: Shape: Linear Trigonal Planar (Flat) Tetrahedral Trigonal Bipyramid Octahedral

Planar triangular Trigonal bipyramidal Tetrahedral Octahedral

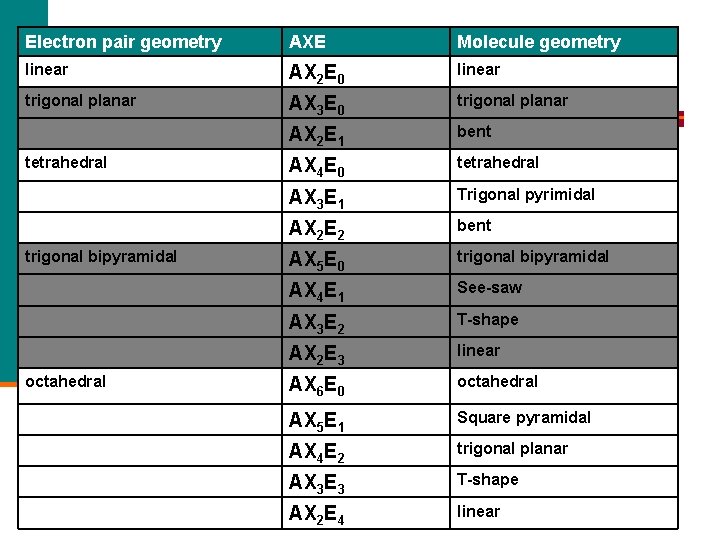
AXE n Lewis structures do not show geometry, only electron pair placement. n However, the 3 -D shape (geometry) of a molecule can be determined from a properly -drawn Lewis structure. n All monocentric molecules can be represented by an AXE formula: A = central atom n X = outer atoms (doesn’t matter what they actually are or how many bonds they are held by) n E = lone pairs of electrons on the central atom only. n

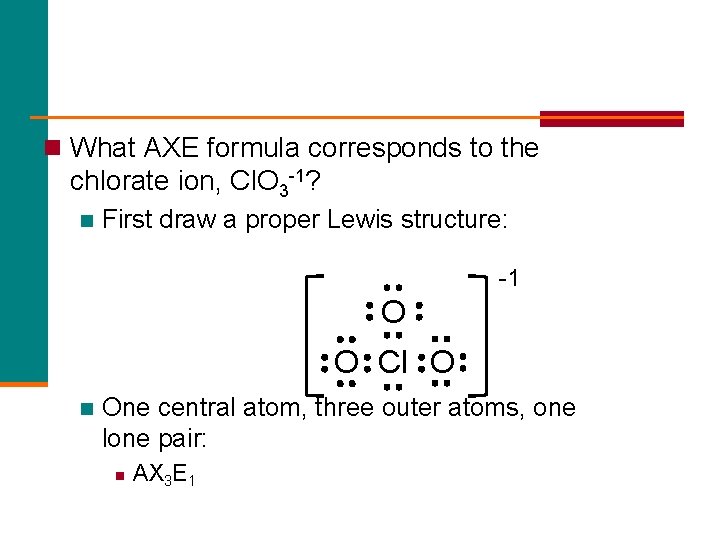
n What AXE formula corresponds to the chlorate ion, Cl. O 3 -1? n First draw a proper Lewis structure: -1 O O Cl O n One central atom, three outer atoms, one lone pair: n AX 3 E 1

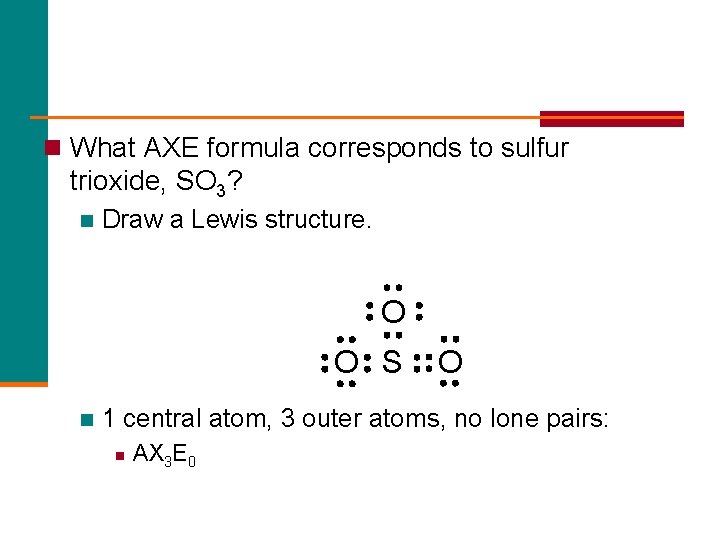
n What AXE formula corresponds to sulfur trioxide, SO 3? n Draw a Lewis structure. O O S n O 1 central atom, 3 outer atoms, no lone pairs: n AX 3 E 0

Electron pair geometry AXE Molecule geometry linear AX 2 E 0 linear trigonal planar AX 3 E 0 trigonal planar AX 2 E 1 bent AX 4 E 0 tetrahedral AX 3 E 1 Trigonal pyrimidal AX 2 E 2 bent AX 5 E 0 trigonal bipyramidal AX 4 E 1 See-saw AX 3 E 2 T-shape AX 2 E 3 linear AX 6 E 0 octahedral AX 5 E 1 Square pyramidal AX 4 E 2 trigonal planar AX 3 E 3 T-shape AX 2 E 4 linear tetrahedral trigonal bipyramidal octahedral

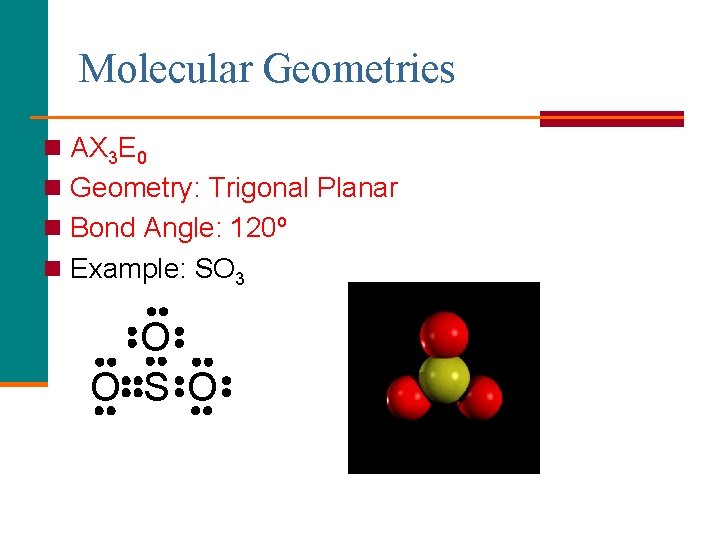
Molecular Geometries n AX 3 E 0 n Geometry: Trigonal Planar n Bond Angle: 120º n Example: SO 3 O O S O

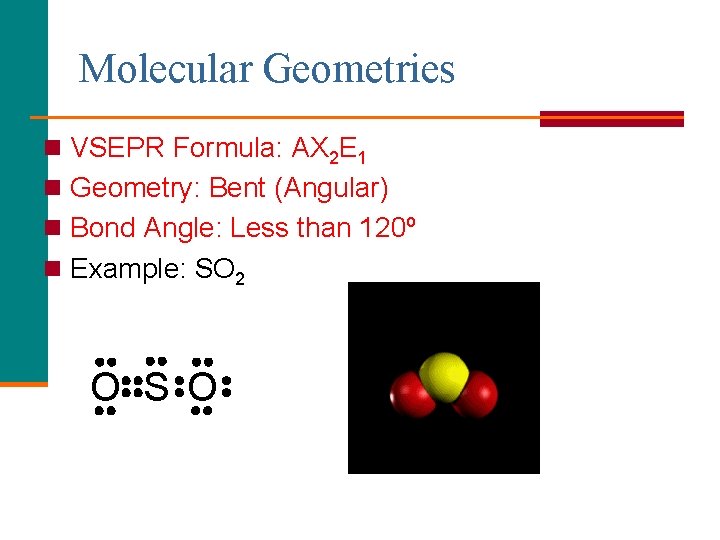
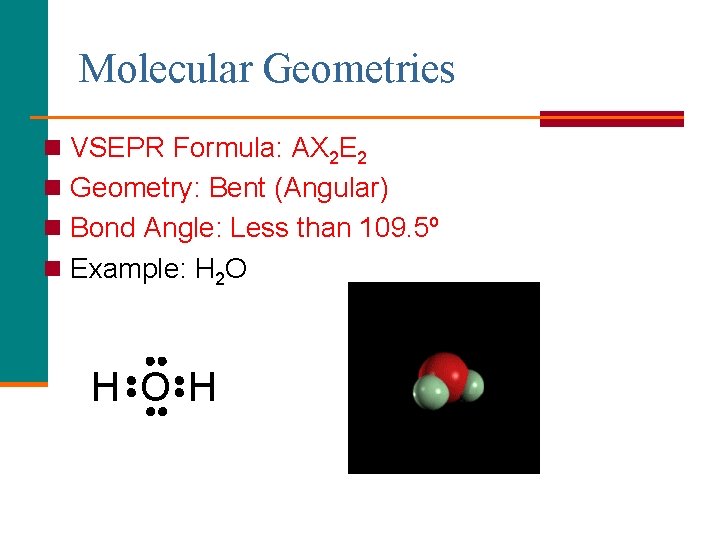
Molecular Geometries n VSEPR Formula: AX 2 E 1 n Geometry: Bent (Angular) n Bond Angle: Less than 120º n Example: SO 2 O S O

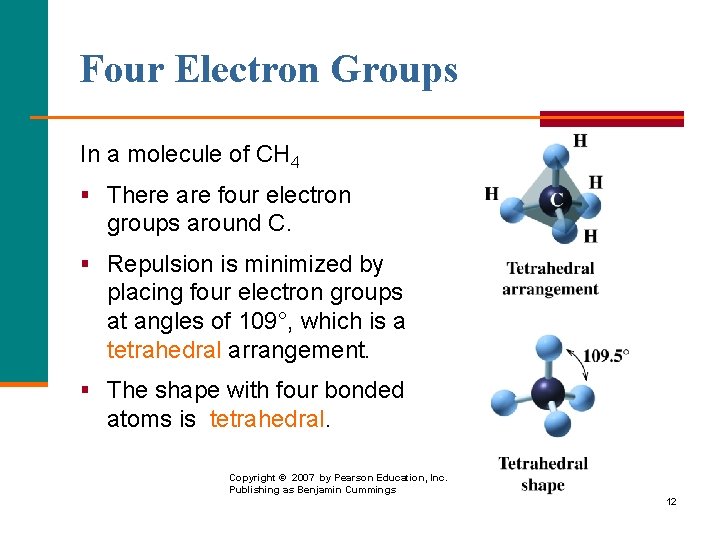
Four Electron Groups In a molecule of CH 4 § There are four electron groups around C. § Repulsion is minimized by placing four electron groups at angles of 109°, which is a tetrahedral arrangement. § The shape with four bonded atoms is tetrahedral. Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings 12

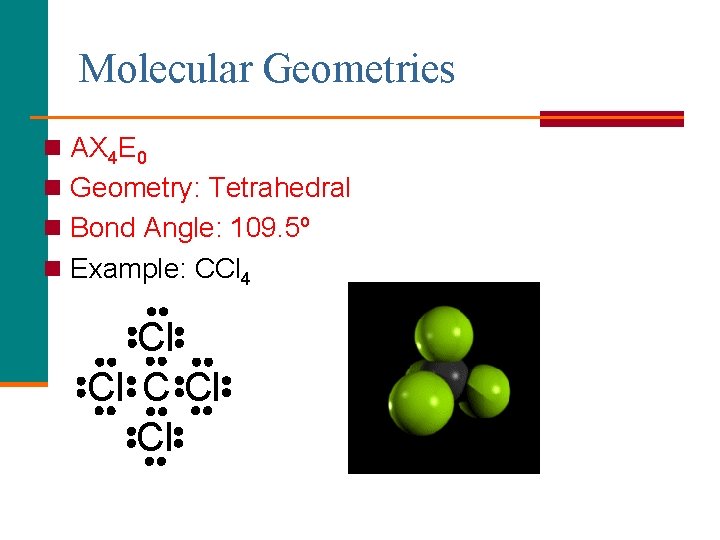
Molecular Geometries n AX 4 E 0 n Geometry: Tetrahedral n Bond Angle: 109. 5º n Example: CCl 4 Cl Cl Cl

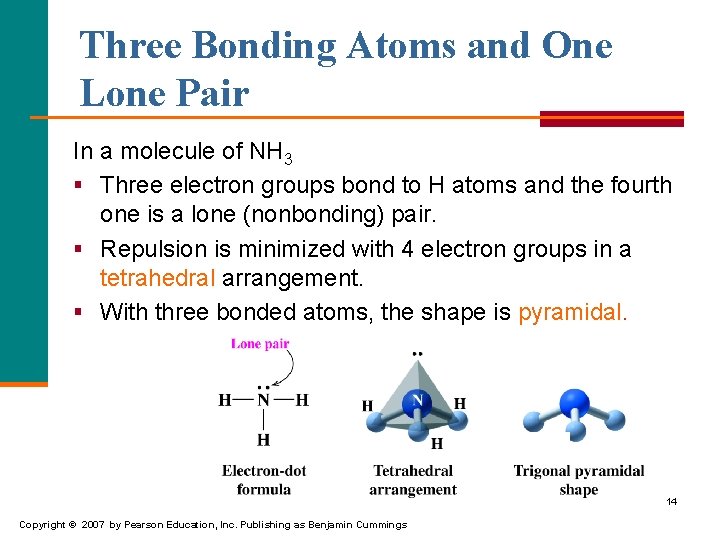
Three Bonding Atoms and One Lone Pair In a molecule of NH 3 § Three electron groups bond to H atoms and the fourth one is a lone (nonbonding) pair. § Repulsion is minimized with 4 electron groups in a tetrahedral arrangement. § With three bonded atoms, the shape is pyramidal. 14 Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings

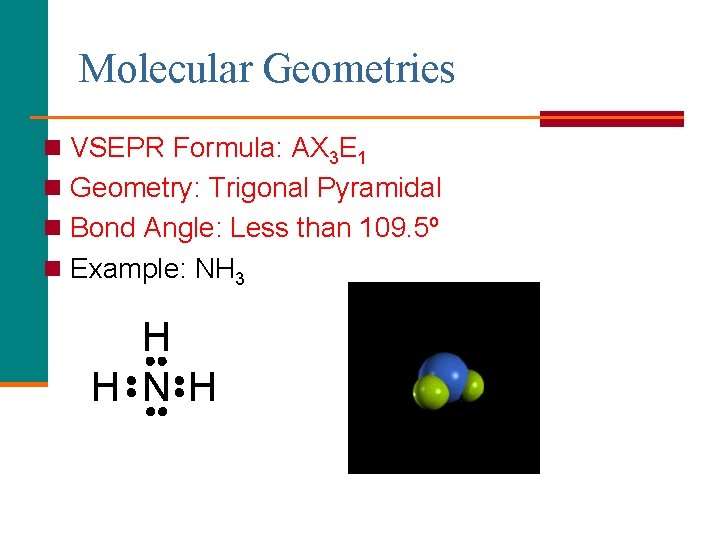
Molecular Geometries n VSEPR Formula: AX 3 E 1 n Geometry: Trigonal Pyramidal n Bond Angle: Less than 109. 5º n Example: NH 3 H H N H

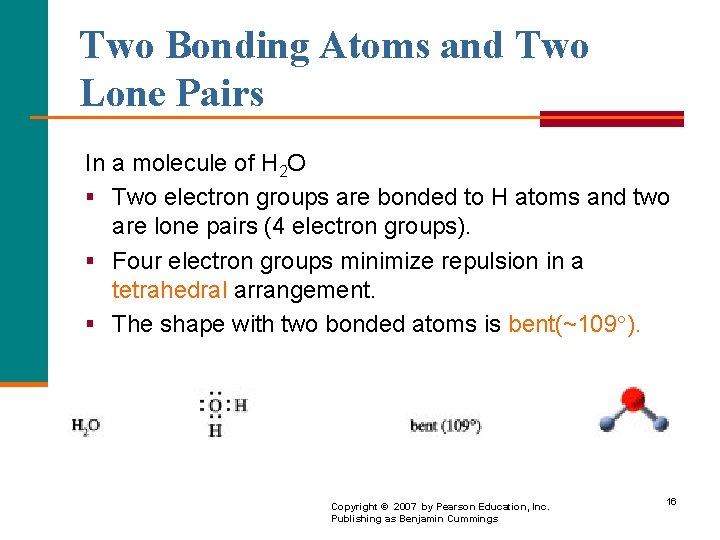
Two Bonding Atoms and Two Lone Pairs In a molecule of H 2 O § Two electron groups are bonded to H atoms and two are lone pairs (4 electron groups). § Four electron groups minimize repulsion in a tetrahedral arrangement. § The shape with two bonded atoms is bent(~109 ). Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings 16

Molecular Geometries n VSEPR Formula: AX 2 E 2 n Geometry: Bent (Angular) n Bond Angle: Less than 109. 5º n Example: H 2 O H

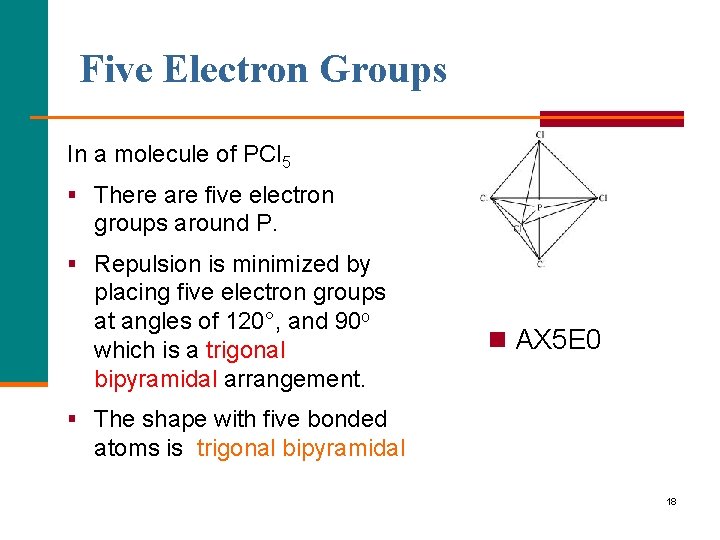
Five Electron Groups In a molecule of PCl 5 § There are five electron groups around P. § Repulsion is minimized by placing five electron groups at angles of 120°, and 90 o which is a trigonal bipyramidal arrangement. n AX 5 E 0 § The shape with five bonded atoms is trigonal bipyramidal 18

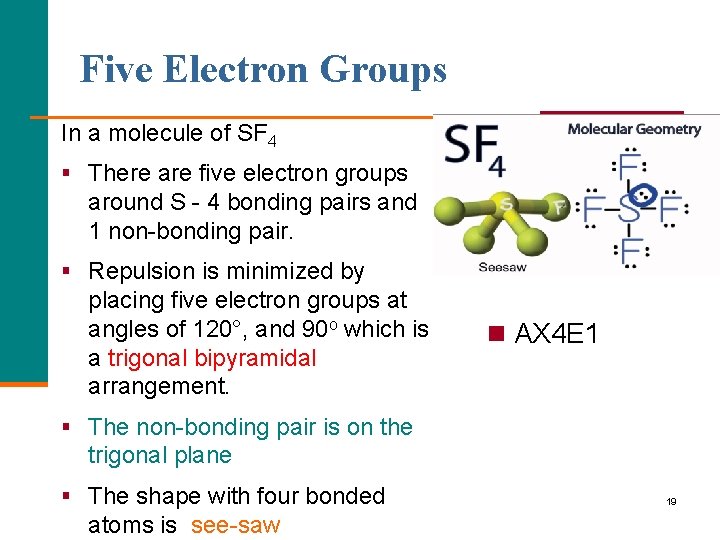
Five Electron Groups In a molecule of SF 4 § There are five electron groups around S - 4 bonding pairs and 1 non-bonding pair. § Repulsion is minimized by placing five electron groups at angles of 120°, and 90 o which is a trigonal bipyramidal arrangement. n AX 4 E 1 § The non-bonding pair is on the trigonal plane § The shape with four bonded atoms is see-saw 19

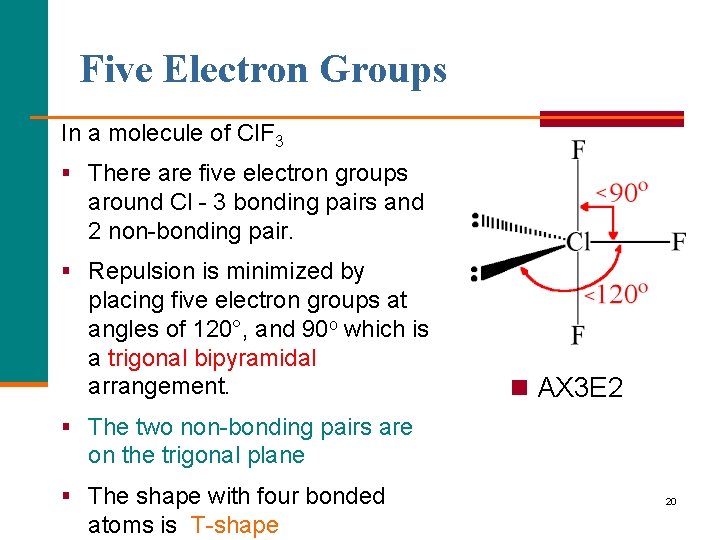
Five Electron Groups In a molecule of Cl. F 3 § There are five electron groups around Cl - 3 bonding pairs and 2 non-bonding pair. § Repulsion is minimized by placing five electron groups at angles of 120°, and 90 o which is a trigonal bipyramidal arrangement. n AX 3 E 2 § The two non-bonding pairs are on the trigonal plane § The shape with four bonded atoms is T-shape 20

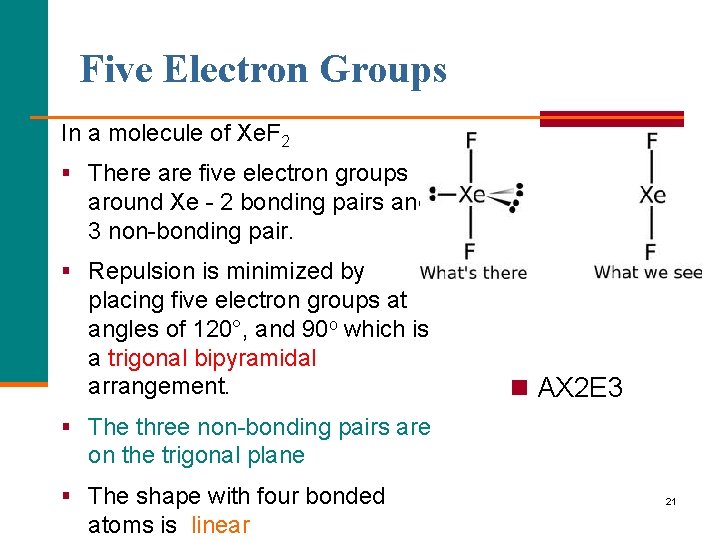
Five Electron Groups In a molecule of Xe. F 2 § There are five electron groups around Xe - 2 bonding pairs and 3 non-bonding pair. § Repulsion is minimized by placing five electron groups at angles of 120°, and 90 o which is a trigonal bipyramidal arrangement. n AX 2 E 3 § The three non-bonding pairs are on the trigonal plane § The shape with four bonded atoms is linear 21

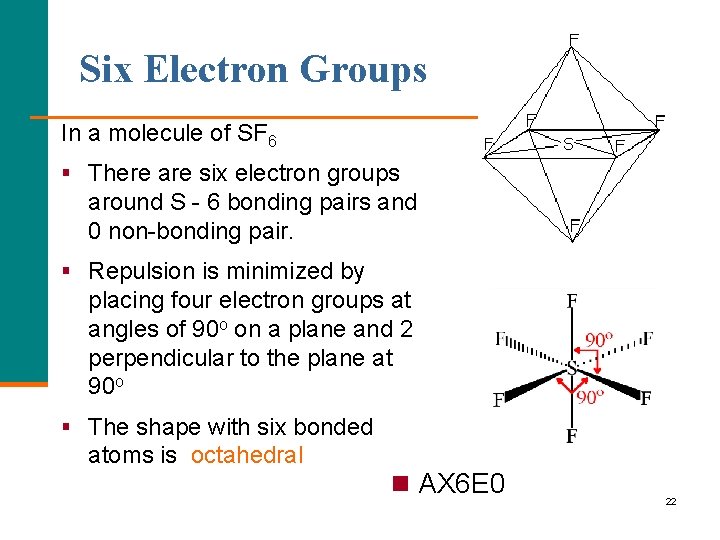
Six Electron Groups In a molecule of SF 6 § There are six electron groups around S - 6 bonding pairs and 0 non-bonding pair. § Repulsion is minimized by placing four electron groups at angles of 90 o on a plane and 2 perpendicular to the plane at 90 o § The shape with six bonded atoms is octahedral n AX 6 E 0 22

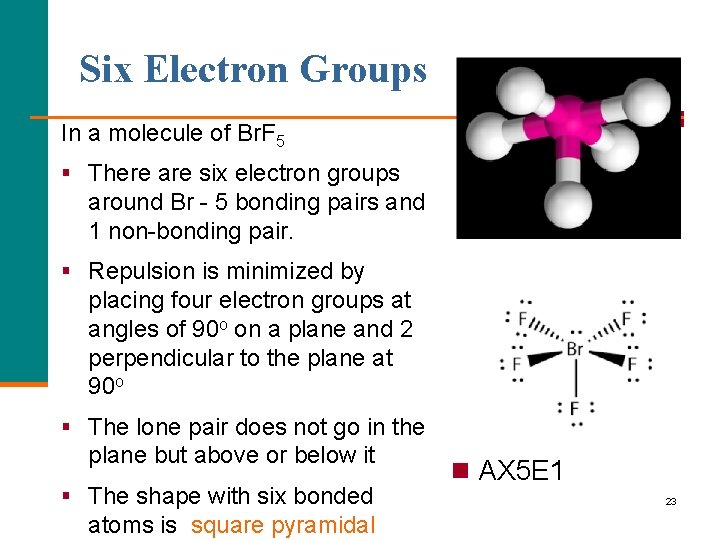
Six Electron Groups In a molecule of Br. F 5 § There are six electron groups around Br - 5 bonding pairs and 1 non-bonding pair. § Repulsion is minimized by placing four electron groups at angles of 90 o on a plane and 2 perpendicular to the plane at 90 o § The lone pair does not go in the plane but above or below it § The shape with six bonded atoms is square pyramidal n AX 5 E 1 23

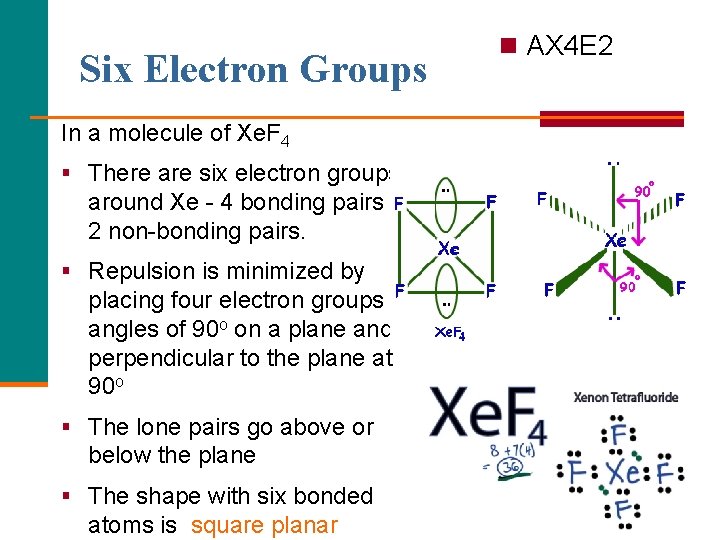
Six Electron Groups n AX 4 E 2 In a molecule of Xe. F 4 § There are six electron groups around Xe - 4 bonding pairs and 2 non-bonding pairs. § Repulsion is minimized by placing four electron groups at angles of 90 o on a plane and 2 perpendicular to the plane at 90 o § The lone pairs go above or below the plane § The shape with six bonded atoms is square planar 24

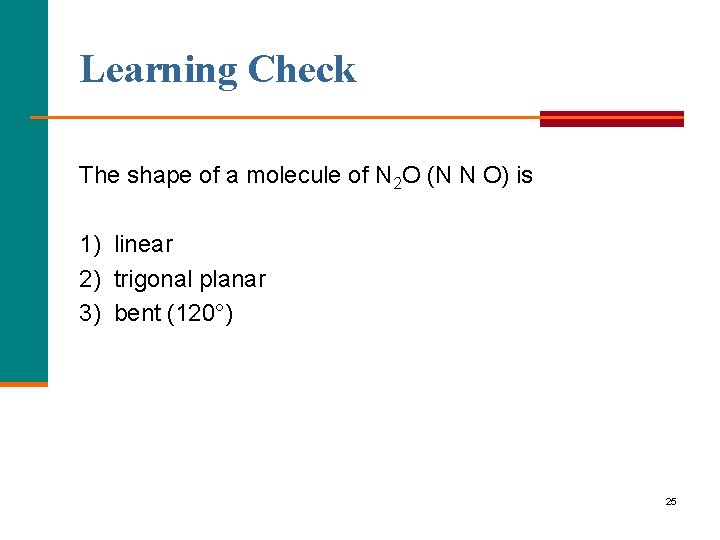
Learning Check The shape of a molecule of N 2 O (N N O) is 1) linear 2) trigonal planar 3) bent (120°) 25

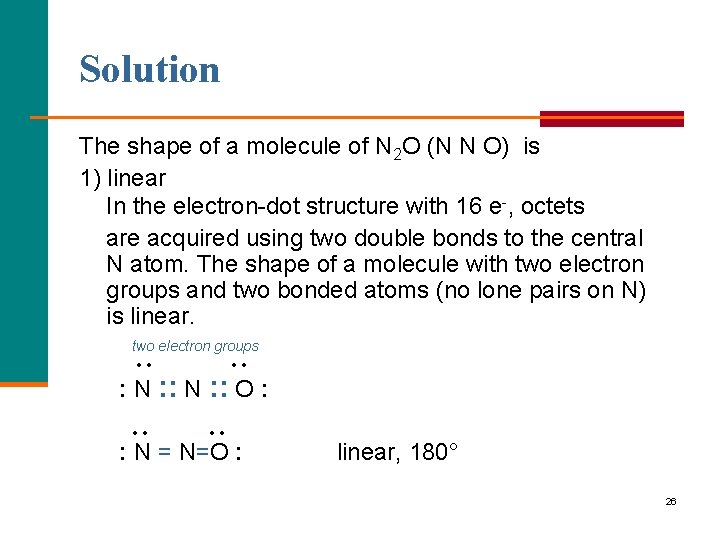
Solution The shape of a molecule of N 2 O (N N O) is 1) linear In the electron-dot structure with 16 e-, octets are acquired using two double bonds to the central N atom. The shape of a molecule with two electron groups and two bonded atoms (no lone pairs on N) is linear. two electron groups • • : N : : O : • • : N = N=O : linear, 180° 26

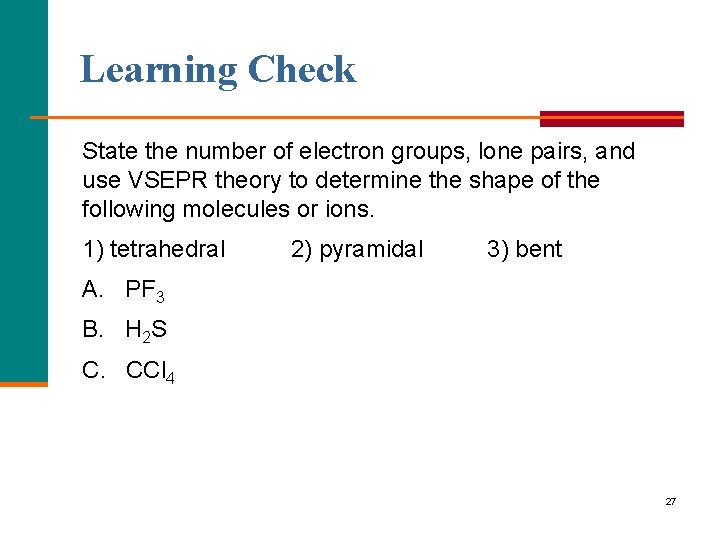
Learning Check State the number of electron groups, lone pairs, and use VSEPR theory to determine the shape of the following molecules or ions. 1) tetrahedral 2) pyramidal 3) bent A. PF 3 B. H 2 S C. CCl 4 27

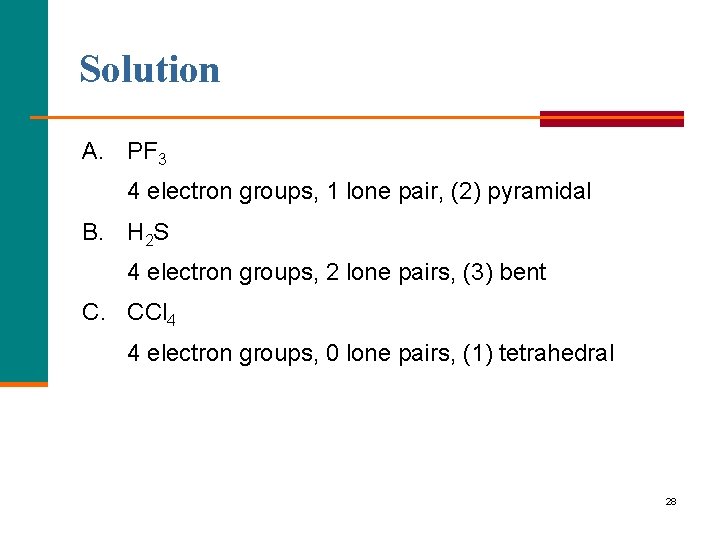
Solution A. PF 3 4 electron groups, 1 lone pair, (2) pyramidal B. H 2 S 4 electron groups, 2 lone pairs, (3) bent C. CCl 4 4 electron groups, 0 lone pairs, (1) tetrahedral 28

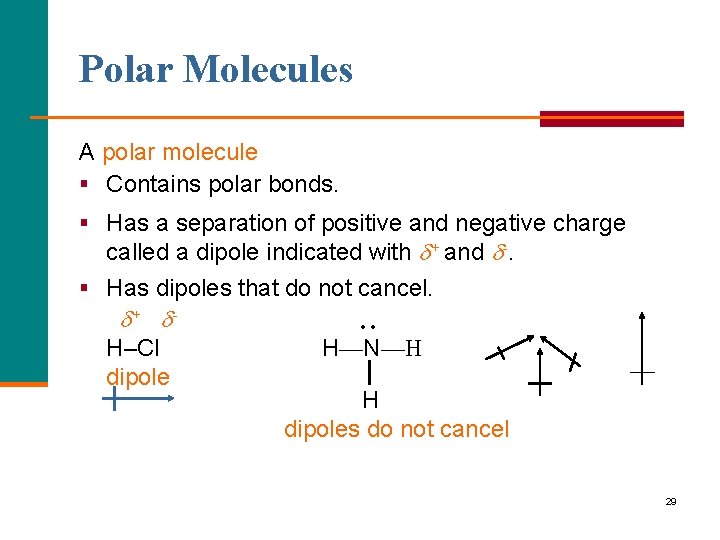
Polar Molecules A polar molecule § Contains polar bonds. § Has a separation of positive and negative charge called a dipole indicated with + and -. § Has dipoles that do not cancel. + H–Cl dipole • • H—N—H H dipoles do not cancel 29

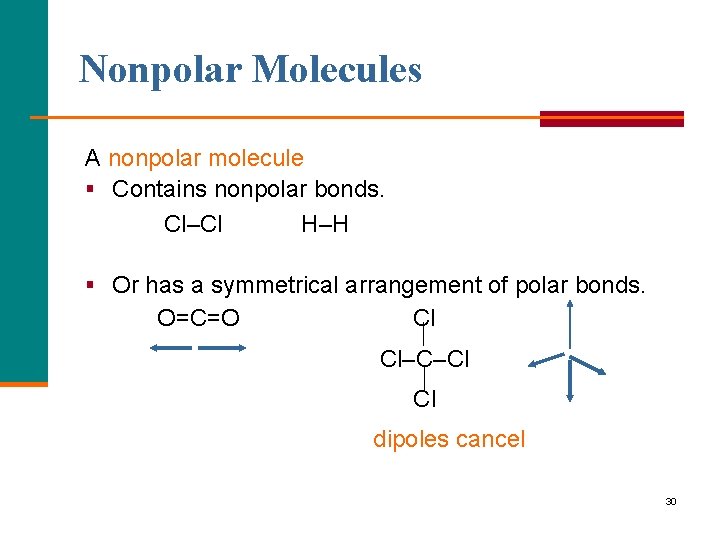
Nonpolar Molecules A nonpolar molecule § Contains nonpolar bonds. Cl–Cl H–H § Or has a symmetrical arrangement of polar bonds. O=C=O Cl Cl–C–Cl Cl dipoles cancel 30

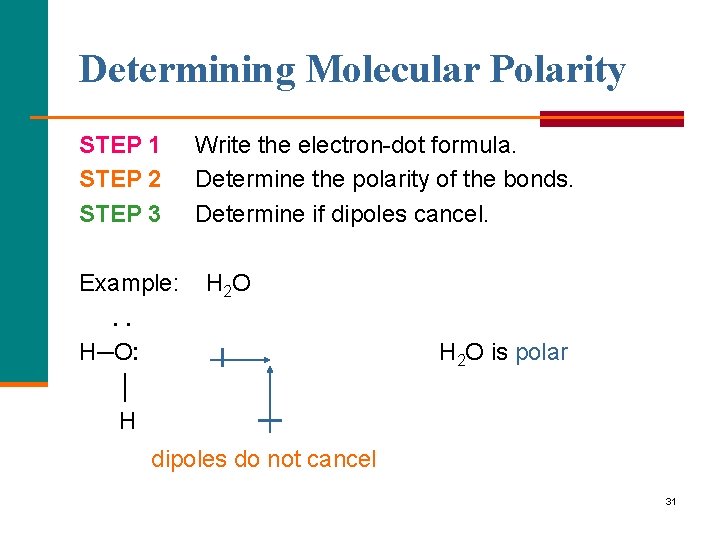
Determining Molecular Polarity STEP 1 STEP 2 STEP 3 Example: . . H─O: │ H Write the electron-dot formula. Determine the polarity of the bonds. Determine if dipoles cancel. H 2 O is polar dipoles do not cancel 31

Learning Check Identify each of the following molecules as 1) polar or 2) nonpolar. Explain. A. PBr 3 B. HBr C. Br 2 D. Si. Br 4 32

Solution Identify each of the following molecules as 1) polar or 2) nonpolar. Explain. A. B. C. D. PBr 3 HBr Br 2 Si. Br 4 1) pyramidal; dipoles don’t cancel; polar 1) linear; one polar bond (dipole); polar 2) linear; nonpolar bond; nonpolar 2) tetrahedral; dipoles cancel; nonpolar 33