VSEPR HYBRIDIZATION Two Theories Which Reinforce Each Other

VSEPR & HYBRIDIZATION
Two Theories Which Reinforce Each Other • VSEPR is essentially theory which helps to explain molecular geometry (shape, and bond angle), because it accounts for the repulsive interaction(s) between the bonded and/or un-bonded electrons of the valence shell. • Hybridization is theory which refines G. N. Lewis’s Valence Bond Theory. VSEPR fails to predict certain issues (such as the bond angle in water molecules). Hybridization explains the experimental data, indicating equal bond energies and bond lengths involving a central atom, and the common atoms to which it is bonded, despite the fact that the bonding electrons are of different energies (because they are were from different sublevels). • Hybridization supports, and is supported by, VSEPR theory and
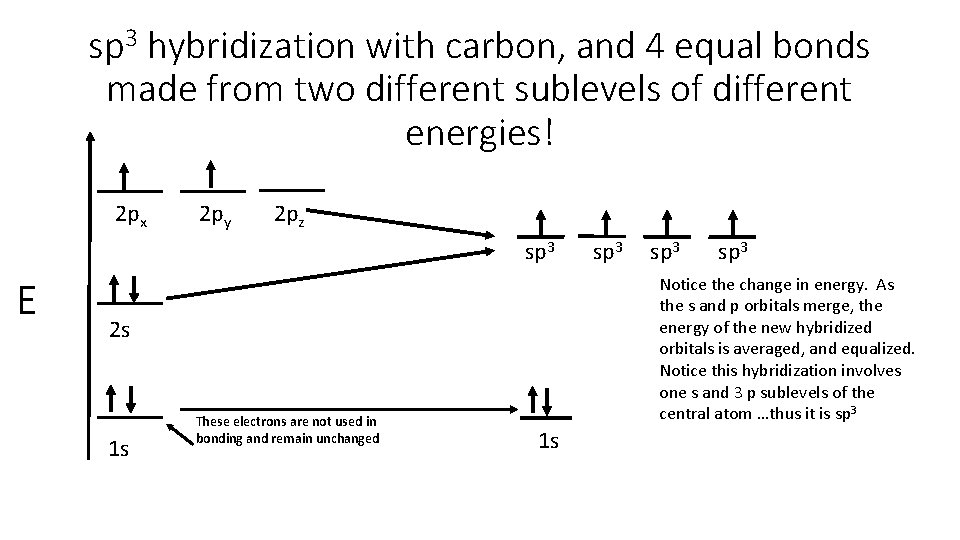
sp 3 hybridization with carbon, and 4 equal bonds made from two different sublevels of different energies! 2 px 2 py 2 pz sp 3 E 1 s sp 3 Notice the change in energy. As the s and p orbitals merge, the energy of the new hybridized orbitals is averaged, and equalized. Notice this hybridization involves one s and 3 p sublevels of the central atom …thus it is sp 3 2 s These electrons are not used in bonding and remain unchanged sp 3 1 s
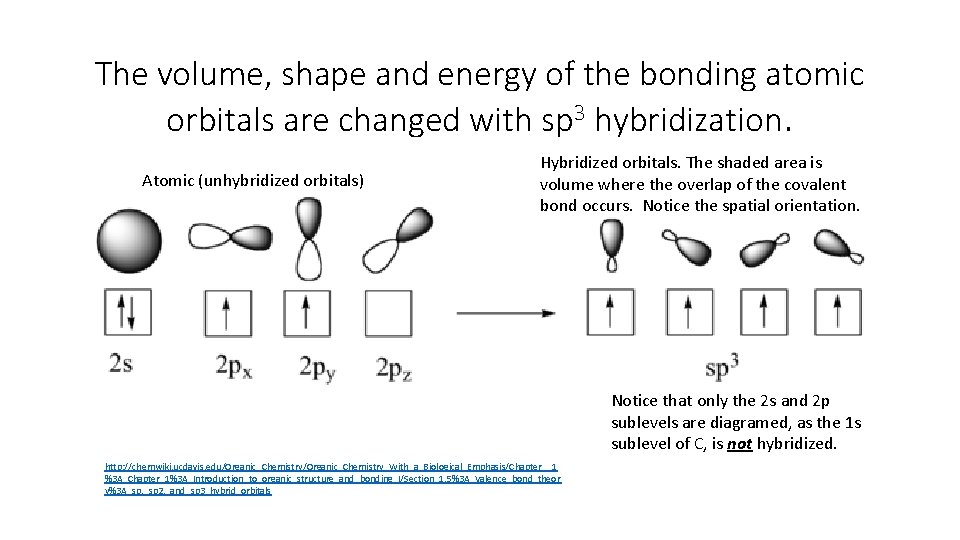
The volume, shape and energy of the bonding atomic orbitals are changed with sp 3 hybridization. Atomic (unhybridized orbitals) Hybridized orbitals. The shaded area is volume where the overlap of the covalent bond occurs. Notice the spatial orientation. Notice that only the 2 s and 2 p sublevels are diagramed, as the 1 s sublevel of C, is not hybridized. http: //chemwiki. ucdavis. edu/Organic_Chemistry_With_a_Biological_Emphasis/Chapter__1 %3 A_Chapter_1%3 A_Introduction_to_organic_structure_and_bonding_I/Section_1. 5%3 A_Valence_bond_theor y%3 A_sp, _sp 2, _and_sp 3_hybrid_orbitals
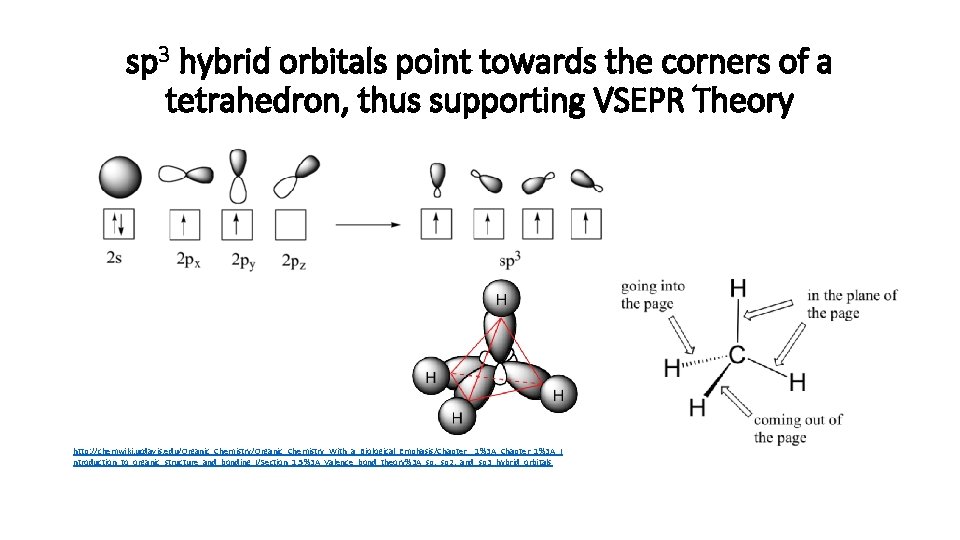
sp 3 hybrid orbitals point towards the corners of a tetrahedron, thus supporting VSEPR Theory http: //chemwiki. ucdavis. edu/Organic_Chemistry_With_a_Biological_Emphasis/Chapter__1%3 A_Chapter_1%3 A_I ntroduction_to_organic_structure_and_bonding_I/Section_1. 5%3 A_Valence_bond_theory%3 A_sp, _sp 2, _and_sp 3_hybrid_orbitals
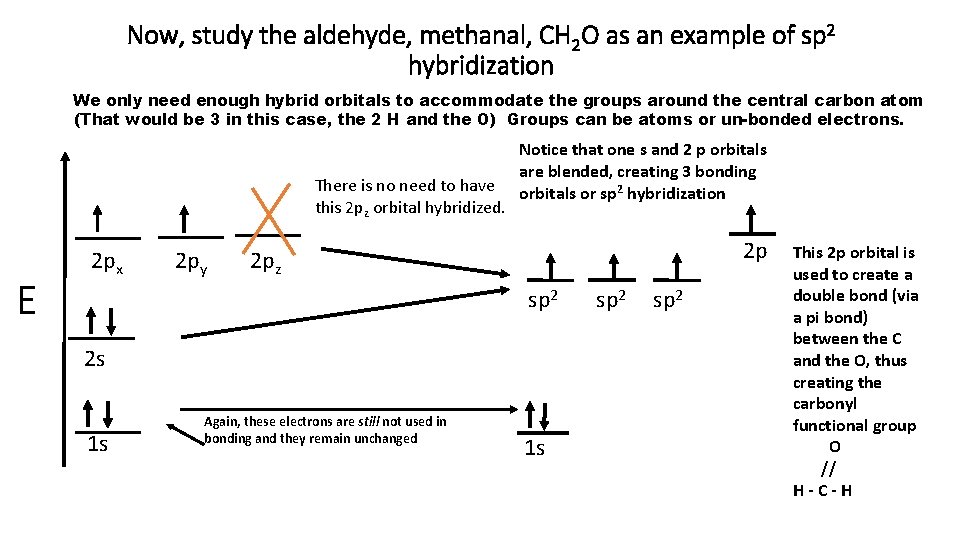
Now, study the aldehyde, methanal, CH 2 O as an example of sp 2 hybridization We only need enough hybrid orbitals to accommodate the groups around the central carbon atom (That would be 3 in this case, the 2 H and the O) Groups can be atoms or un-bonded electrons. There is no need to have this 2 pz orbital hybridized. E 2 px 2 py Notice that one s and 2 p orbitals are blended, creating 3 bonding orbitals or sp 2 hybridization 2 p 2 pz sp 2 2 s 1 s Again, these electrons are still not used in bonding and they remain unchanged 1 s sp 2 This 2 p orbital is used to create a double bond (via a pi bond) between the C and the O, thus creating the carbonyl functional group O // H-C-H
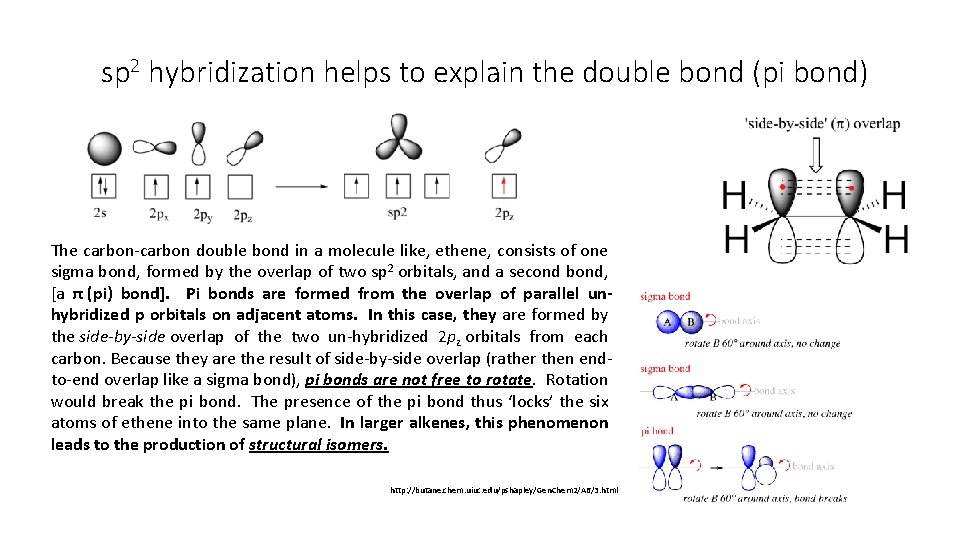
sp 2 hybridization helps to explain the double bond (pi bond) The carbon-carbon double bond in a molecule like, ethene, consists of one sigma bond, formed by the overlap of two sp 2 orbitals, and a second bond, [a π (pi) bond]. Pi bonds are formed from the overlap of parallel unhybridized p orbitals on adjacent atoms. In this case, they are formed by the side-by-side overlap of the two un-hybridized 2 pz orbitals from each carbon. Because they are the result of side-by-side overlap (rather then endto-end overlap like a sigma bond), pi bonds are not free to rotate. Rotation would break the pi bond. The presence of the pi bond thus ‘locks’ the six atoms of ethene into the same plane. In larger alkenes, this phenomenon leads to the production of structural isomers. http: //butane. chem. uiuc. edu/pshapley/Gen. Chem 2/A 6/3. html
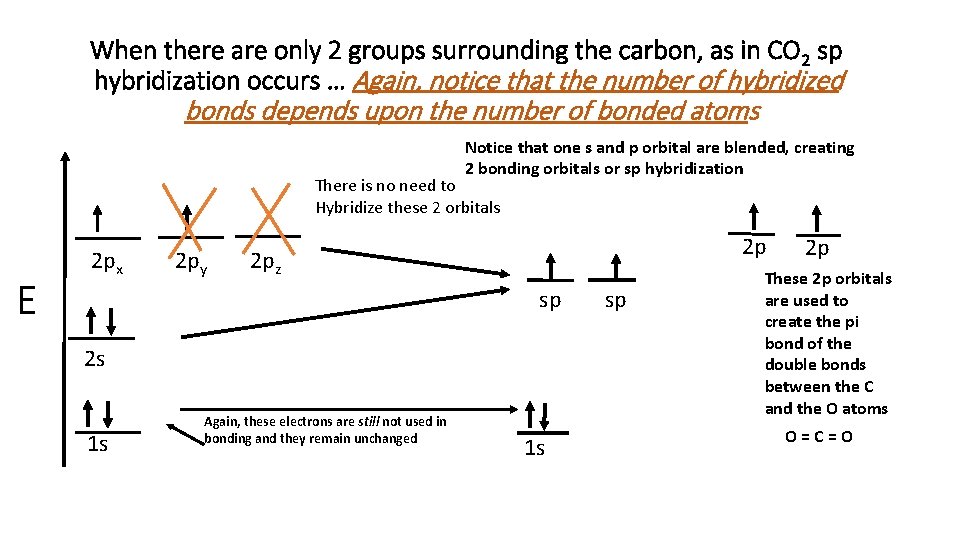
When there are only 2 groups surrounding the carbon, as in CO 2 sp hybridization occurs … Again, notice that the number of hybridized bonds depends upon the number of bonded atoms Notice that one s and p orbital are blended, creating 2 bonding orbitals or sp hybridization There is no need to Hybridize these 2 orbitals E 2 px 2 py 2 p 2 pz sp 2 s 1 s Again, these electrons are still not used in bonding and they remain unchanged 1 s sp 2 p These 2 p orbitals are used to create the pi bond of the double bonds between the C and the O atoms O=C=O
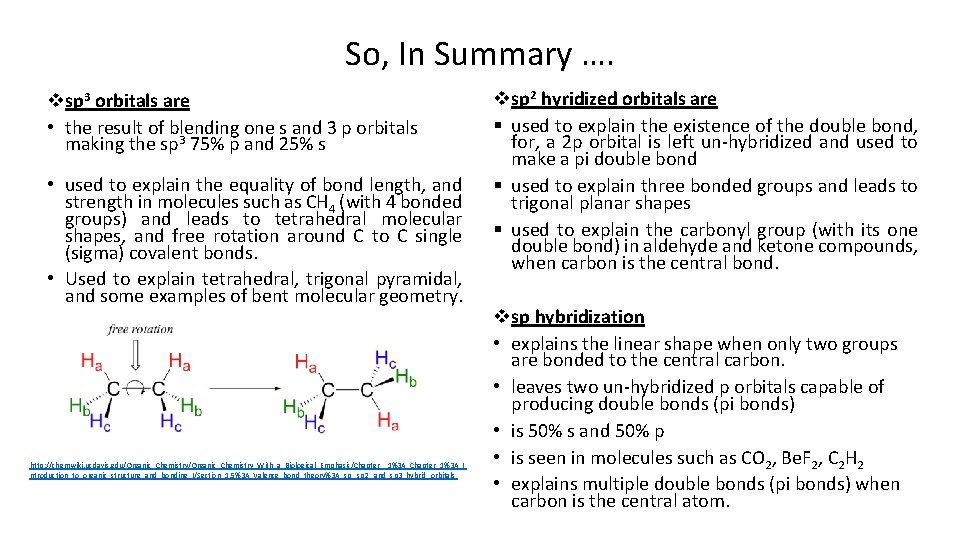
So, In Summary …. vsp 3 orbitals are • the result of blending one s and 3 p orbitals making the sp 3 75% p and 25% s • used to explain the equality of bond length, and strength in molecules such as CH 4 (with 4 bonded groups) and leads to tetrahedral molecular shapes, and free rotation around C to C single (sigma) covalent bonds. • Used to explain tetrahedral, trigonal pyramidal, and some examples of bent molecular geometry. http: //chemwiki. ucdavis. edu/Organic_Chemistry_With_a_Biological_Emphasis/Chapter__1%3 A_Chapter_1%3 A_I ntroduction_to_organic_structure_and_bonding_I/Section_1. 5%3 A_Valence_bond_theory%3 A_sp, _sp 2, _and_sp 3_hybrid_orbitals vsp 2 hyridized orbitals are § used to explain the existence of the double bond, for, a 2 p orbital is left un-hybridized and used to make a pi double bond § used to explain three bonded groups and leads to trigonal planar shapes § used to explain the carbonyl group (with its one double bond) in aldehyde and ketone compounds, when carbon is the central bond. vsp hybridization • explains the linear shape when only two groups are bonded to the central carbon. • leaves two un-hybridized p orbitals capable of producing double bonds (pi bonds) • is 50% s and 50% p • is seen in molecules such as CO 2, Be. F 2, C 2 H 2 • explains multiple double bonds (pi bonds) when carbon is the central atom.

To help understand sigma and pi bonds a bit better, try these two videos …come in with questions, tomorrow! • Try this quirky 5 minute video with some very nice points to make about sigma and pi bonds, and hybridization. • Check out the whole video, but take a special look at around 3: 58 for a nice little clay model view of pi bond formation. http: //www. youtube. com/watch? v=ree 49 ge 4 VA 4 • Per usual Crash Course Chemistry is fast paced series - but each installment has some nice points to make. You might want to stop and replay a section now and then. • This 11 minute video has some special points for review and edification: • at 3: 48 you will get an review at to why water is a polar molecule and at • 7: 50 there is a really nice visualization of pi bond formation. http: //www. youtube. com/watch? v=c. PDptc 0 w. UYI

Citations Based upon ideas found at or in: 1. Morrison and Boyd: Organic Chemistry 2. Pauling, L: General Chemistry 3. http: //www. chem. uiuc. edu/CLCtutorials/104/Hybridiz ation/See. It. html 4. http: //chemwiki. ucdavis. edu/Organic_Chemistry/Orga nic_Chemistry_With_a_Biological_Emphasis/Chapter_ _1%3 A_Chapter_1%3 A_Introduction_to_organic_struc ture_and_bonding_I/Section_1. 5%3 A_Valence_bond_ theory%3 A_sp, _sp 2, _and_sp 3_hybrid_orbitals 5. http: //butane. chem. uiuc. edu/pshapley/Gen. Chem 2/A 6 /3. html 6. https: //en. wikipedia. org/wiki/Pi_bond
- Slides: 11