VOYAGER PAD Efficacy and Safety of Rivaroxaban in

VOYAGER PAD Efficacy and Safety of Rivaroxaban in Patients with Symptomatic PAD undergoing Revascularization with and without Clopidogrel William R. Hiatt, Marc P. Bonaca*, Manesh R. Patel, Mark R. Nehler, Eike Sebastian Debus, Sonia S. Anand, Warren H Capell, Lihong Diao, Nicole Jaeger, Connie N. Hess, Akos Ferenc Pap, Scott D. Berkowitz, Eva Muehlhofer, Lloyd Haskell, David Brasil, Juraf Madaric, Henrick Sillesen, David Szalay, Rupert Bauersachs on behalf of the VOYAGER PAD Investigators American College of Cardiology Virtual Scientific Sessions 2020 Late-Breaking Clinical Trial *Drs. Hiatt and Bonaca Contributed Equally to this Presentation 29 March 2020 An Academic Research Organization Affiliated with the University of Colorado School of Medicine

William R Hiatt Disclosures Research grants to CPC Clinical Research, an Academic Research Organization and Affiliate of the University of Colorado Anschutz Campus • Bayer • Janssen • Amgen 2
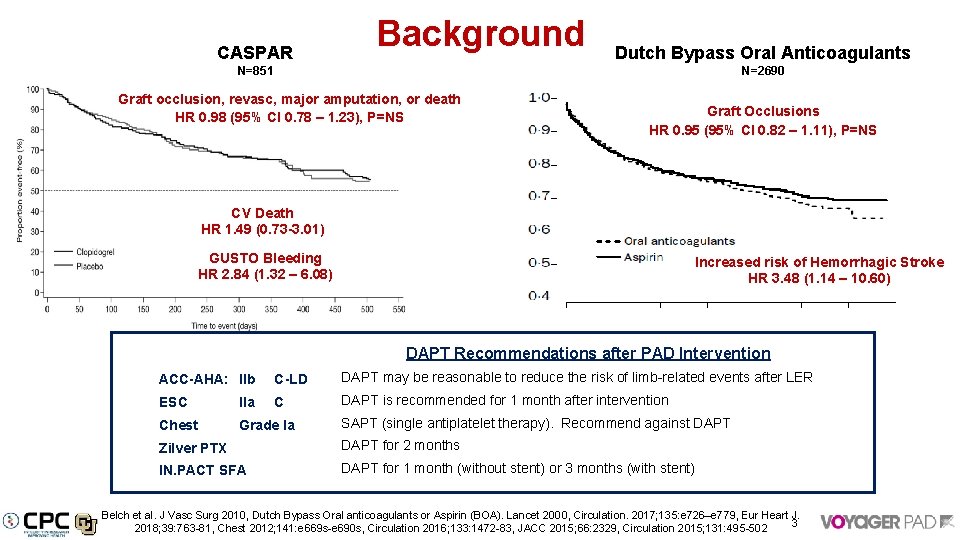
CASPAR Background Dutch Bypass Oral Anticoagulants N=851 N=2690 Graft occlusion, revasc, major amputation, or death HR 0. 98 (95% CI 0. 78 – 1. 23), P=NS Graft Occlusions HR 0. 95 (95% CI 0. 82 – 1. 11), P=NS CV Death HR 1. 49 (0. 73 -3. 01) GUSTO Bleeding HR 2. 84 (1. 32 – 6. 08) Increased risk of Hemorrhagic Stroke HR 3. 48 (1. 14 – 10. 60) DAPT Recommendations after PAD Intervention ACC-AHA: IIb C-LD DAPT may be reasonable to reduce the risk of limb-related events after LER ESC IIa C DAPT is recommended for 1 month after intervention Chest Grade Ia SAPT (single antiplatelet therapy). Recommend against DAPT Zilver PTX DAPT for 2 months IN. PACT SFA DAPT for 1 month (without stent) or 3 months (with stent) Belch et al. J Vasc Surg 2010, Dutch Bypass Oral anticoagulants or Aspirin (BOA). Lancet 2000, Circulation. 2017; 135: e 726–e 779, Eur Heart J. 3 2018; 39: 763 -81, Chest 2012; 141: e 669 s-e 690 s, Circulation 2016; 133: 1472 -83, JACC 2015; 66: 2329, Circulation 2015; 131: 495 -502
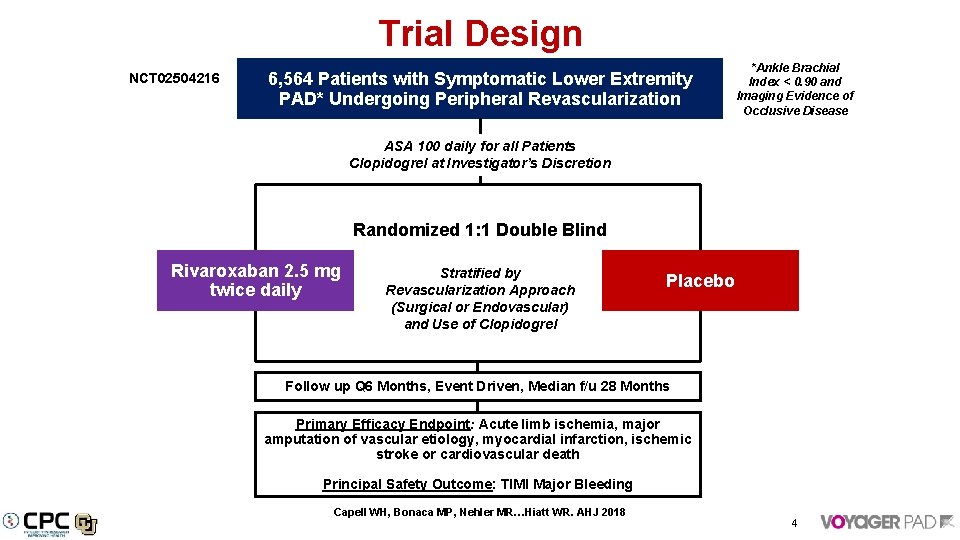
Trial Design NCT 02504216 6, 564 Patients with Symptomatic Lower Extremity PAD* Undergoing Peripheral Revascularization *Ankle Brachial Index < 0. 90 and Imaging Evidence of Occlusive Disease ASA 100 daily for all Patients Clopidogrel at Investigator’s Discretion Randomized 1: 1 Double Blind Rivaroxaban 2. 5 mg twice daily Stratified by Revascularization Approach (Surgical or Endovascular) and Use of Clopidogrel Placebo Follow up Q 6 Months, Event Driven, Median f/u 28 Months Primary Efficacy Endpoint: Acute limb ischemia, major amputation of vascular etiology, myocardial infarction, ischemic stroke or cardiovascular death Principal Safety Outcome: TIMI Major Bleeding Capell WH, Bonaca MP, Nehler MR…Hiatt WR. AHJ 2018 4

Inclusion & Exclusion Inclusion • Age ≥ 50 • Documented PAD including: • • Ischemic symptoms (functional limitation, rest pain or ischemic ulceration) AND • Imaging evidence of occlusion AND • Abnormal ABI Successful lower extremity revascularization for ischemia • Revascularization for asymptomatic disease • Recent revascularization (within 10 days) or ALI (2 weeks) or ACS (30 days) • Current major tissue loss • Need for antiplatelet or anticoagulant other than aspirin and/or clopidogrel • Need for long-term DAPT (intended > 6 months) • High risk for bleeding (significant bleeding in last 6 months, prior stroke or other highrisk condition) 5
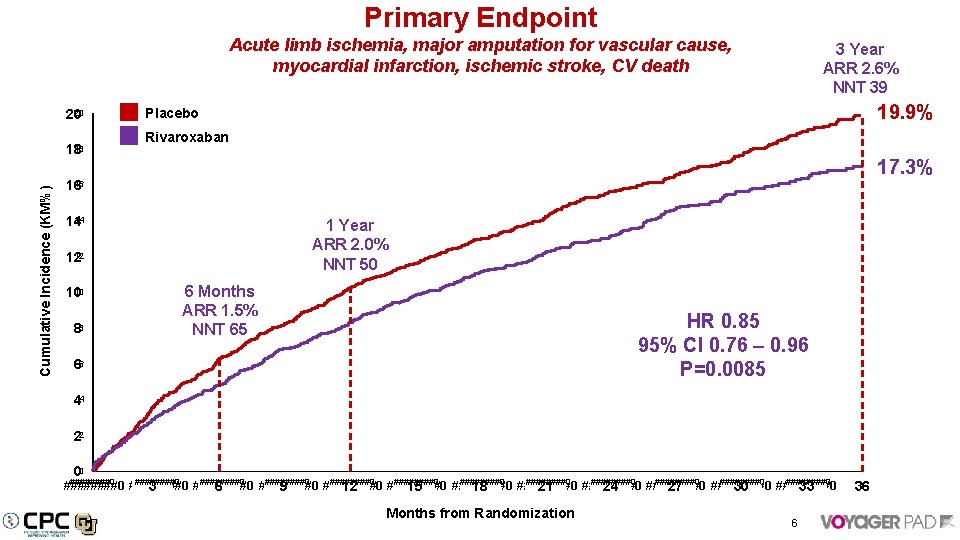
Primary Endpoint Acute limb ischemia, major amputation for vascular cause, myocardial infarction, ischemic stroke, CV death 20 20 Cumulative Incidence (KM%) 18 18 3 Year ARR 2. 6% NNT 39 19. 9% Placebo Rivaroxaban 17. 3% 16 16 14 14 1 Year ARR 2. 0% NNT 50 12 12 10 10 88 6 Months ARR 1. 5% NNT 65 HR 0. 85 95% CI 0. 76 – 0. 96 P=0. 0085 66 44 22 00 ########0 ########0 ########0 ########0 ########0 ########0 3 6 9 12 15 18 21 24 27 30 33 Months from Randomization 6 36

Objectives In symptomatic PAD patients undergoing lower extremity revascularization randomized to rivaroxaban 2. 5 mg twice daily with aspirin versus aspirin alone, to evaluate whether: • Determine if efficacy and safety of rivaroxaban were consistent regardless of background clopidogrel use • To explore temporal patterns of bleeding in relationship to exposure and duration of clopidogrel 7
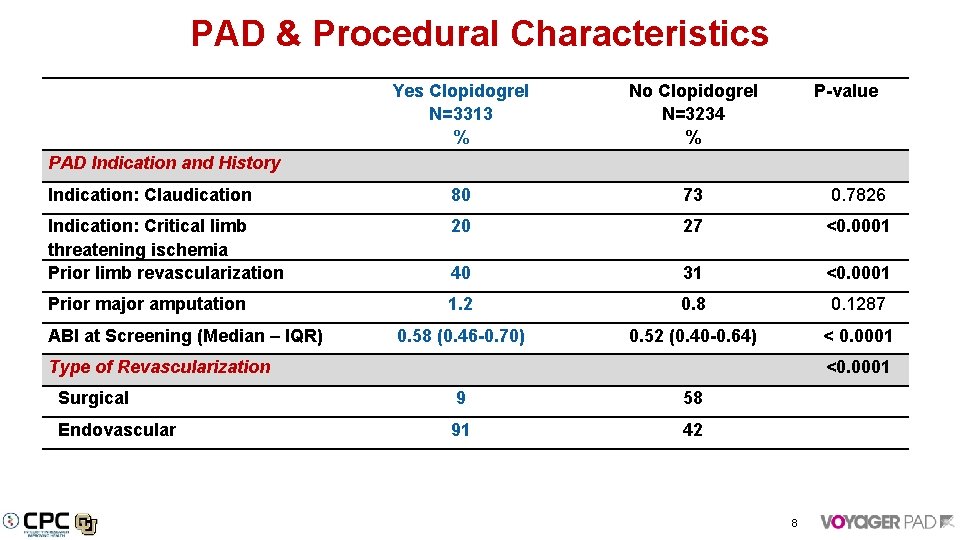
PAD & Procedural Characteristics PAD Indication and History Yes Clopidogrel N=3313 % No Clopidogrel N=3234 % P-value Indication: Claudication 80 73 0. 7826 Indication: Critical limb threatening ischemia Prior limb revascularization 20 27 <0. 0001 40 31 <0. 0001 Prior major amputation 1. 2 0. 8 0. 1287 0. 58 (0. 46 -0. 70) 0. 52 (0. 40 -0. 64) < 0. 0001 Type of Revascularization <0. 0001 Surgical 9 58 Endovascular 91 42 ABI at Screening (Median – IQR) 8
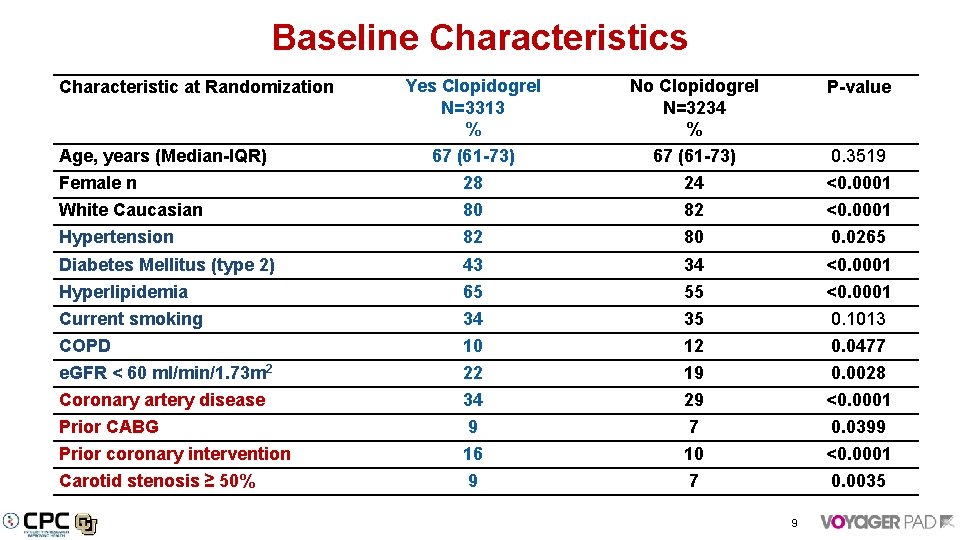
Baseline Characteristics Yes Clopidogrel N=3313 % 67 (61 -73) No Clopidogrel N=3234 % 67 (61 -73) P-value Female n 28 24 <0. 0001 White Caucasian 80 82 <0. 0001 Hypertension 82 80 0. 0265 Diabetes Mellitus (type 2) 43 34 <0. 0001 Hyperlipidemia 65 55 <0. 0001 Current smoking 34 35 0. 1013 COPD 10 12 0. 0477 e. GFR < 60 ml/min/1. 73 m 2 22 19 0. 0028 Coronary artery disease 34 29 <0. 0001 Prior CABG 9 7 0. 0399 Prior coronary intervention 16 10 <0. 0001 Carotid stenosis ≥ 50% 9 7 0. 0035 Characteristic at Randomization Age, years (Median-IQR) 0. 3519 9
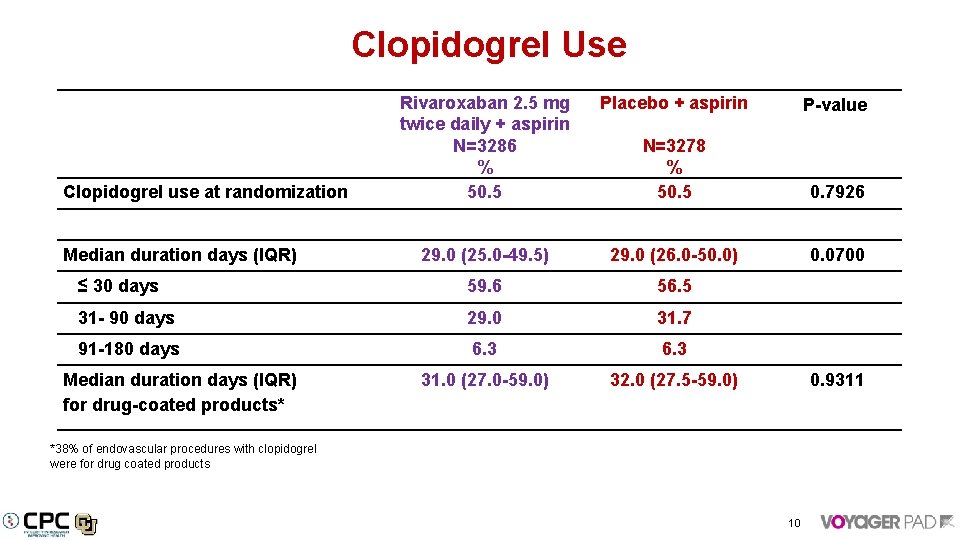
Clopidogrel Use Rivaroxaban 2. 5 mg twice daily + aspirin N=3286 % 50. 5 Placebo + aspirin P-value N=3278 % 50. 5 0. 7926 29. 0 (25. 0 -49. 5) 29. 0 (26. 0 -50. 0) 0. 0700 ≤ 30 days 59. 6 56. 5 31 - 90 days 29. 0 31. 7 91 -180 days 6. 3 31. 0 (27. 0 -59. 0) 32. 0 (27. 5 -59. 0) 0. 9311 Clopidogrel use at randomization Median duration days (IQR) for drug-coated products* *38% of endovascular procedures with clopidogrel were for drug coated products 10
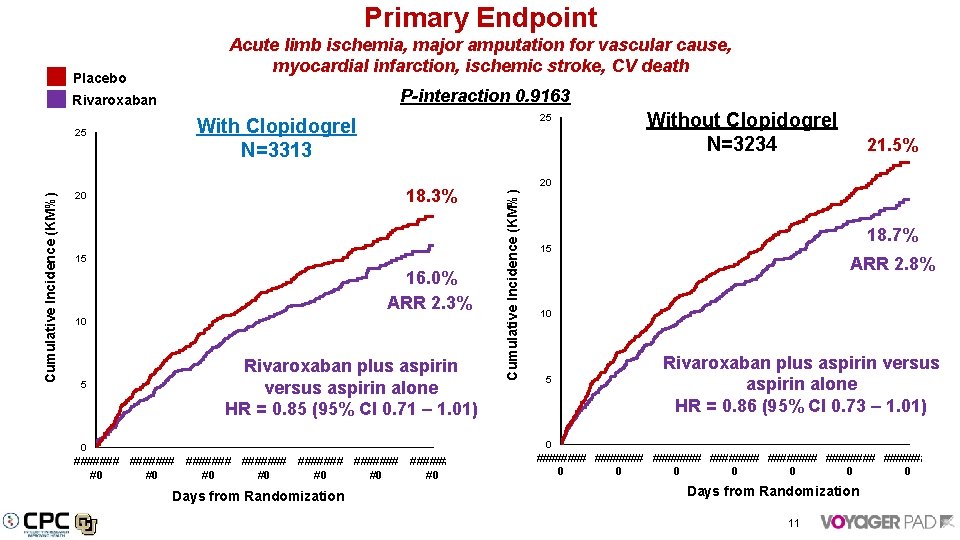
Primary Endpoint P-interaction 0. 9163 Rivaroxaban Cumulative Incidence (KM%) 25 25 With Clopidogrel N=3313 18. 3% 20 15 16. 0% ARR 2. 3% 10 5 Rivaroxaban plus aspirin versus aspirin alone HR = 0. 85 (95% CI 0. 71 – 1. 01) 0 ####### ####### #0 #0 Days from Randomization Without Clopidogrel N=3234 21. 5% 20 Cumulative Incidence (KM%) Placebo Acute limb ischemia, major amputation for vascular cause, myocardial infarction, ischemic stroke, CV death 18. 7% 15 ARR 2. 8% 10 5 Rivaroxaban plus aspirin versus aspirin alone HR = 0. 86 (95% CI 0. 73 – 1. 01) 0 ######## ######## 0 0 0 0 Days from Randomization 11
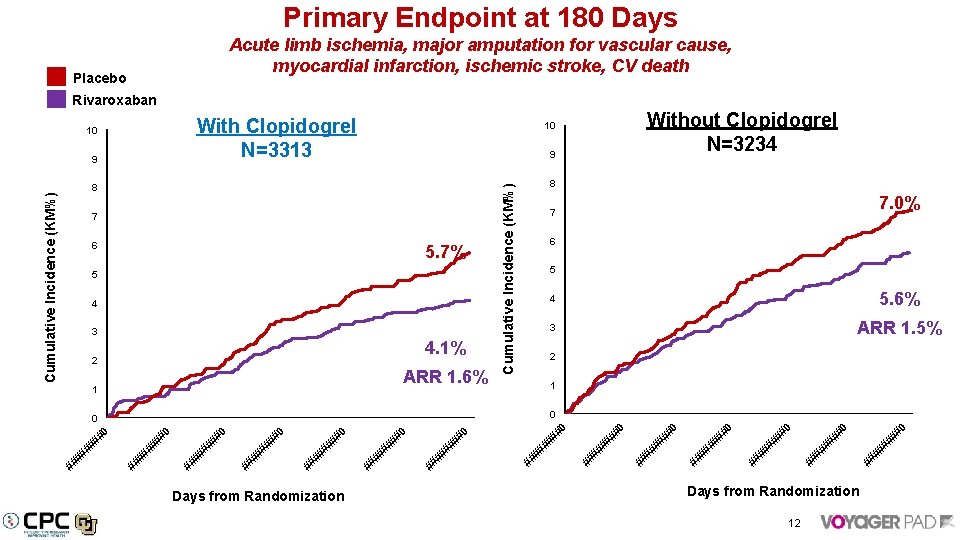
Primary Endpoint at 180 Days Acute limb ischemia, major amputation for vascular cause, myocardial infarction, ischemic stroke, CV death Placebo Rivaroxaban With Clopidogrel N=3313 9 9 8 7 6 5. 7% 5 4 3 4. 1% 2 ARR 1. 6% 1 8 6 5 5. 6% 3 ARR 1. 5% 1 #0 ## ## #0 ## ## ## #0 #0 ## ## ## # #0 ## ## ## # Days from Randomization #0 0 ## # #0 ## ## 4 2 0 ## # 7. 0% 7 ## # Cumulative Incidence (KM%) Without Clopidogrel N=3234 10 Cumulative Incidence (KM%) 10 Days from Randomization 12
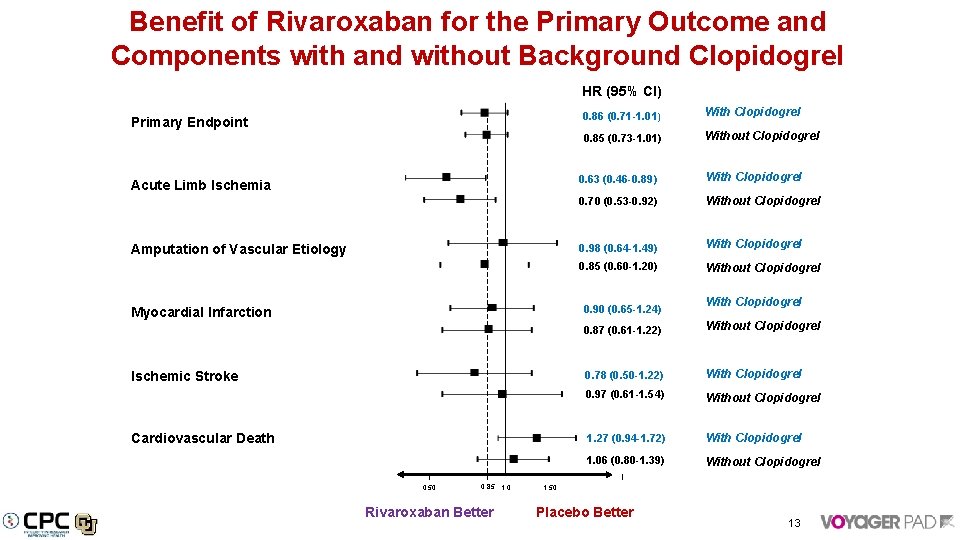
Benefit of Rivaroxaban for the Primary Outcome and Components with and without Background Clopidogrel HR (95% CI) Primary Endpoint Acute Limb Ischemia Amputation of Vascular Etiology 0. 86 (0. 71 -1. 01) With Clopidogrel 0. 85 (0. 73 -1. 01) Without Clopidogrel 0. 63 (0. 46 -0. 89) With Clopidogrel 0. 70 (0. 53 -0. 92) Without Clopidogrel 0. 98 (0. 64 -1. 49) With Clopidogrel 0. 85 (0. 60 -1. 20) Without Clopidogrel 0. 90 (0. 65 -1. 24) Myocardial Infarction Ischemic Stroke Cardiovascular Death 0. 50 0. 85 1. 0 Rivaroxaban Better With Clopidogrel 0. 87 (0. 61 -1. 22) Without Clopidogrel 0. 78 (0. 50 -1. 22) With Clopidogrel 0. 97 (0. 61 -1. 54) Without Clopidogrel 1. 27 (0. 94 -1. 72) With Clopidogrel 1. 06 (0. 80 -1. 39) Without Clopidogrel 1. 50 Placebo Better 13
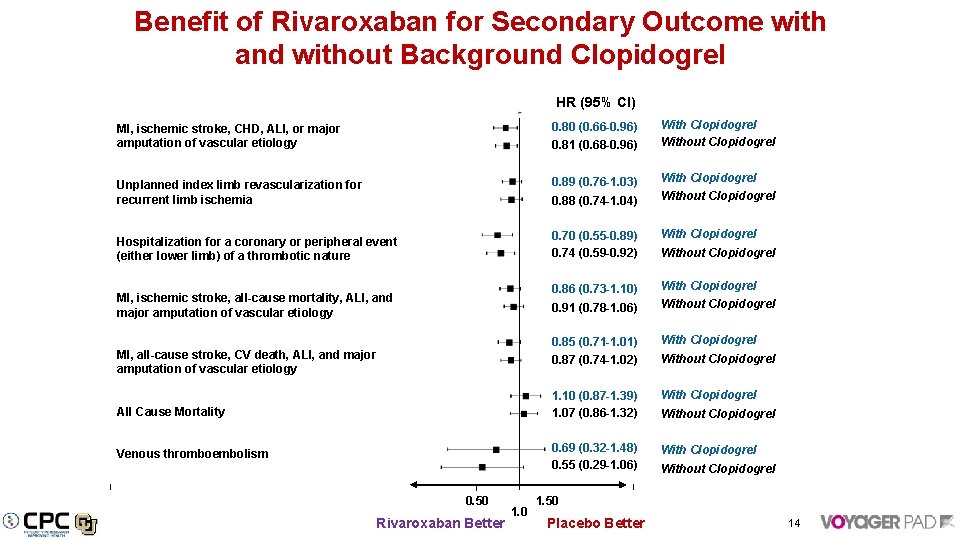
Benefit of Rivaroxaban for Secondary Outcome with and without Background Clopidogrel HR (95% CI) With Clopidogrel Without Clopidogrel MI, ischemic stroke, CHD, ALI, or major amputation of vascular etiology 0. 80 (0. 66 -0. 96) Unplanned index limb revascularization for recurrent limb ischemia 0. 89 (0. 76 -1. 03) 0. 88 (0. 74 -1. 04) Hospitalization for a coronary or peripheral event (either lower limb) of a thrombotic nature 0. 70 (0. 55 -0. 89) 0. 74 (0. 59 -0. 92) With Clopidogrel 0. 86 (0. 73 -1. 10) 0. 91 (0. 78 -1. 06) With Clopidogrel Without Clopidogrel 0. 85 (0. 71 -1. 01) With Clopidogrel 0. 87 (0. 74 -1. 02) Without Clopidogrel 1. 10 (0. 87 -1. 39) 1. 07 (0. 86 -1. 32) With Clopidogrel 0. 69 (0. 32 -1. 48) 0. 55 (0. 29 -1. 06) With Clopidogrel 0. 81 (0. 68 -0. 96) MI, ischemic stroke, all-cause mortality, ALI, and major amputation of vascular etiology MI, all-cause stroke, CV death, ALI, and major amputation of vascular etiology All Cause Mortality Venous thromboembolism 0. 50 Rivaroxaban Better 1. 0 With Clopidogrel Without Clopidogrel 1. 50 Placebo Better 14
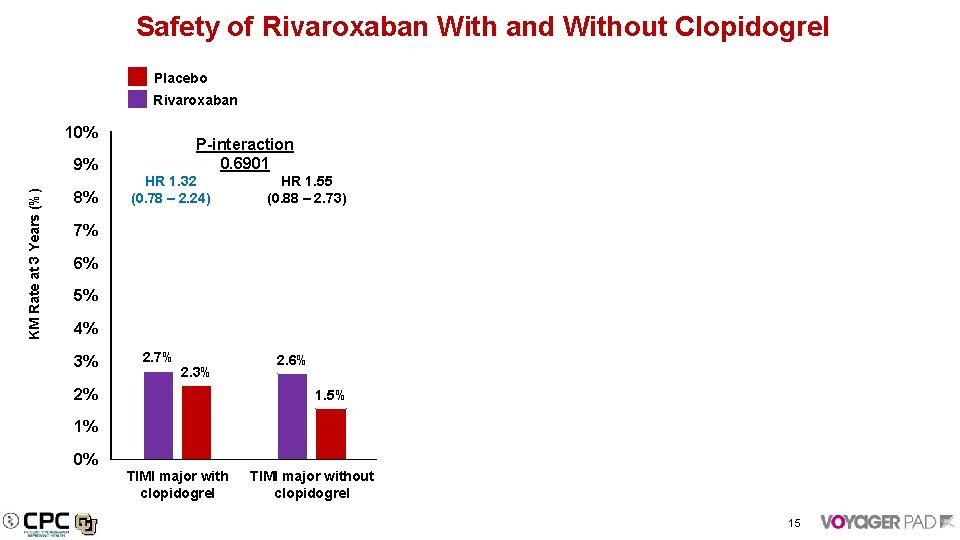
Safety of Rivaroxaban With and Without Clopidogrel Placebo Rivaroxaban 10% KM Rate at 3 Years (%) 9% 8% P-interaction 0. 6901 HR 1. 32 (0. 78 – 2. 24) P-interaction 0. 1261 HR 1. 55 (0. 88 – 2. 73) HR 0. 44 (0. 14 – 1. 43) HR 1. 35 (0. 59 – 3. 07) 7% P-interaction 0. 7002 HR 1. 36 (0. 96 – 1. 91) HR 1. 50 (1. 02 – 2. 20) 6. 5% 6% 5. 4% 4. 9% 5% 4% 3% 3. 3% 2. 7% 2. 3% 2% 2. 6% 1. 5% 1% 1. 2% 0. 8% 0. 3% 0% TIMI major with clopidogrel TIMI major without ICH or Fatal without ISTH major without clopidogrel clopidogrel 15
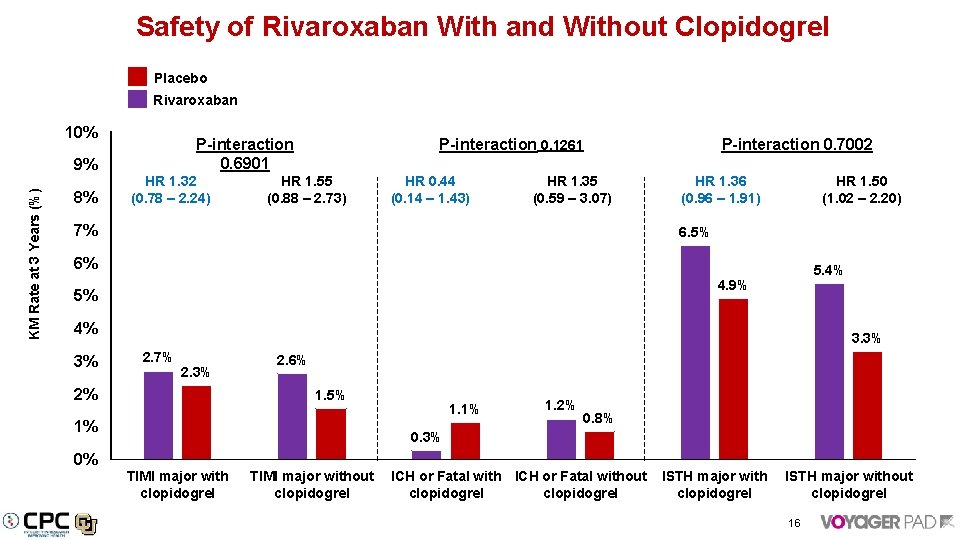
Safety of Rivaroxaban With and Without Clopidogrel Placebo Rivaroxaban 10% KM Rate at 3 Years (%) 9% 8% P-interaction 0. 6901 HR 1. 32 (0. 78 – 2. 24) P-interaction 0. 1261 HR 1. 55 (0. 88 – 2. 73) HR 0. 44 (0. 14 – 1. 43) HR 1. 35 (0. 59 – 3. 07) 7% P-interaction 0. 7002 HR 1. 36 (0. 96 – 1. 91) HR 1. 50 (1. 02 – 2. 20) 6. 5% 6% 5. 4% 4. 9% 5% 4% 3% 3. 3% 2. 7% 2. 3% 2% 2. 6% 1. 5% 1% 1. 2% 0. 8% 0. 3% 0% TIMI major with clopidogrel TIMI major without ICH or Fatal without ISTH major without clopidogrel clopidogrel 16
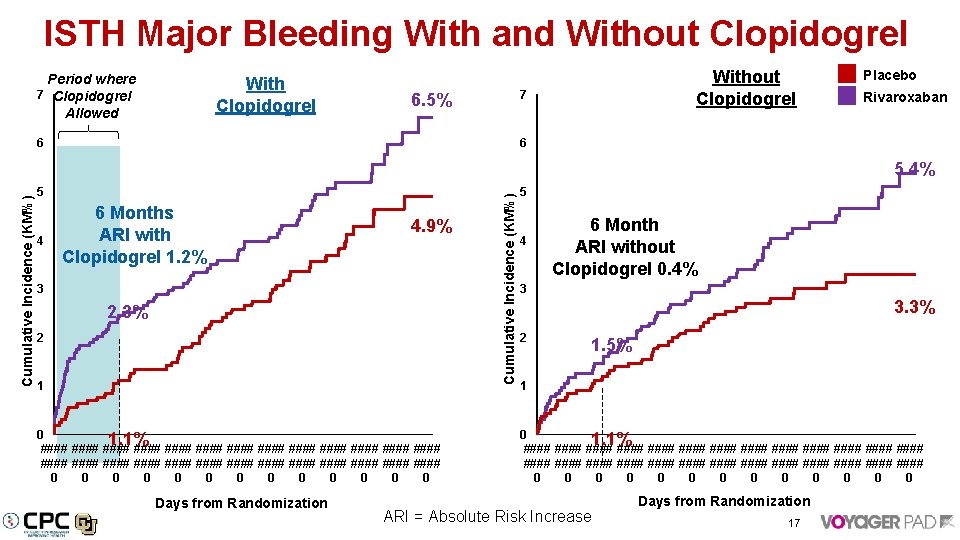
ISTH Major Bleeding With and Without Clopidogrel Period where 7 Clopidogrel Allowed With Clopidogrel Without Clopidogrel 7 6. 5% 6 Placebo Rivaroxaban 6 5 4 6 Months ARI with Clopidogrel 1. 2% 4. 9% 3 2. 3% 2 1 Cumulative Incidence (KM%) 5. 4% 5 4 6 Month ARI without Clopidogrel 0. 4% 3 3. 3% 2 1. 5% 1 0 1. 1% #### #### #### #### #### #### #### #### #### #### #### #### #### 0 0 0 0 0 0 0 Days from Randomization ARI = Absolute Risk Increase 17
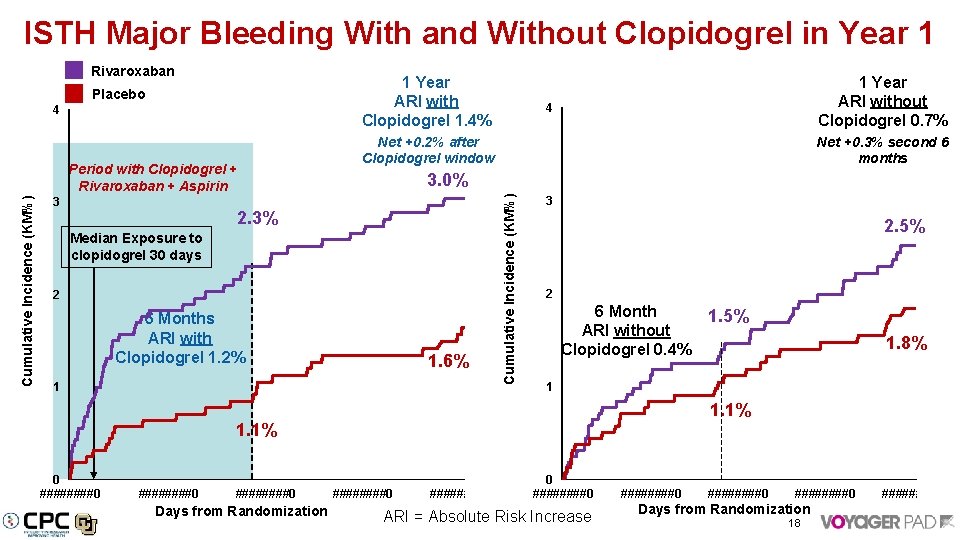
ISTH Major Bleeding With and Without Clopidogrel in Year 1 Rivaroxaban 1 Year ARI with Clopidogrel 1. 4% 4 Cumulative Incidence (KM%) Period with Clopidogrel + Rivaroxaban + Aspirin 3 1 Year ARI without Clopidogrel 0. 7% 4 Net +0. 2% after Clopidogrel window Net +0. 3% second 6 months 3. 0% 2. 3% Median Exposure to clopidogrel 30 days 2 6 Months ARI with Clopidogrel 1. 2% 1. 6% 1 Cumulative Incidence (KM%) Placebo 3 2. 5% 2 6 Month ARI without Clopidogrel 0. 4% ########0 Days from Randomization 1. 8% 1 1. 1% 0 ####0 1. 5% ########0 0 ####0 ARI = Absolute Risk Increase ########0 Days from Randomization 18 ####0
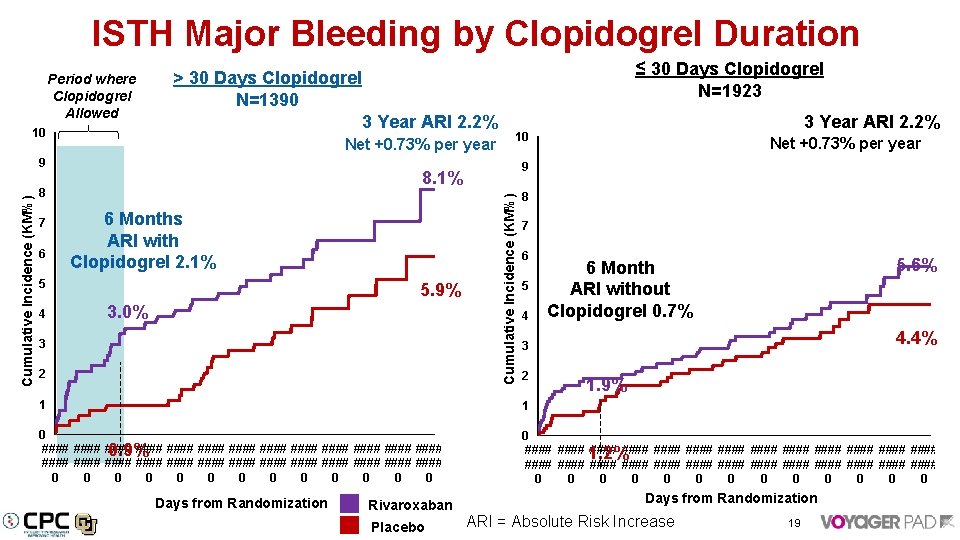
ISTH Major Bleeding by Clopidogrel Duration Period where Clopidogrel Allowed 3 Year ARI 2. 2% 10 Net +0. 73% per year 6 6 Months ARI with Clopidogrel 2. 1% 5 4 5. 9% 3. 0% 3 2 Net +0. 73% per year 9 8. 1% 8 3 Year ARI 2. 2% 10 Cumulative Incidence (KM%) 9 7 ≤ 30 Days Clopidogrel N=1923 > 30 Days Clopidogrel N=1390 8 7 6 5 4 5. 6% 6 Month ARI without Clopidogrel 0. 7% 4. 4% 3 2 1. 9% 1 1 0 #### #### #### #### 0. 9% #### #### #### #### 0 0 0 0 #### 1. 2% #### #### #### #### #### #### 0 0 0 0 Days from Randomization Rivaroxaban Placebo ARI = Absolute Risk Increase 19
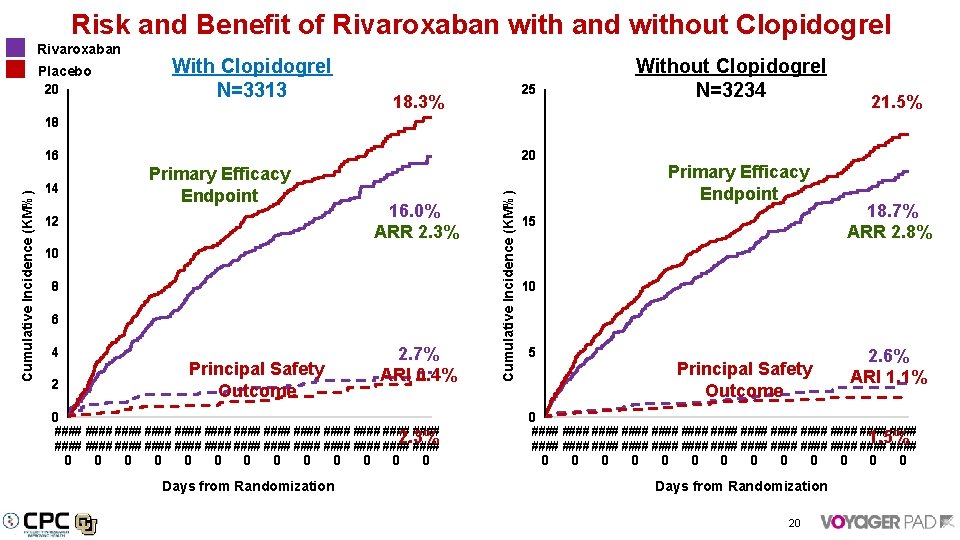
Risk and Benefit of Rivaroxaban with and without Clopidogrel Rivaroxaban Placebo 20 With Clopidogrel N=3313 25 18. 3% Without Clopidogrel N=3234 21. 5% 18 20 14 Primary Efficacy Endpoint 12 16. 0% ARR 2. 3% 10 8 6 4 2 Principal Safety Outcome 2. 7% ARI 0. 4% 0 #### #### #### #### 2. 3% #### #### #### #### 0 0 0 0 Days from Randomization Cumulative Incidence (KM%) 16 Primary Efficacy Endpoint 15 18. 7% ARR 2. 8% 10 5 Principal Safety Outcome 2. 6% ARI 1. 1% 0 #### #### #### #### 1. 5% #### #### #### #### 0 0 0 0 Days from Randomization 20
Summary • In patients with symptomatic PAD undergoing revascularization: – The benefit of rivaroxaban plus aspirin versus aspirin alone is consistent regardless of background clopidogrel • Primary efficacy endpoint HR ~0. 85 with rivaroxaban regardless of clopidogrel with NNT < 50 with or without clopidogrel – The safety of rivaroxaban plus aspirin versus aspirin alone is consistent regardless of background clopidogrel • Principal safety outcome TIMI major bleeding HR ~1. 3 -1. 5 regardless of clopidogrel with NNH > 90 with or without clopidogrel – However, clopidogrel exposure was associated with higher rates of bleeding overall, particularly with longer durations (e. g. > 30 days) 21
Conclusions & Perspective In patients with symptomatic PAD undergoing revascularization: – The benefit of DAPT is uncertain, with the only RCT in surgical bypass showing no benefit and significantly increased bleeding – Rivaroxaban added to aspirin significantly reduces limb and cardiovascular risk with consistent benefits regardless of clopidogrel – The safety and risk/benefit of rivaroxaban plus aspirin are consistent regardless of background clopidogrel – In patients receiving rivaroxaban, the addition of clopidogrel as a third agent, is associated with higher rates of bleeding during exposure – More bleeding with background clopidogrel, even if not severe by adjudication, may be associated with broad consequences, including discontinuation of therapies. In the absence of clear benefit, clopidogrel exposure along with aspirin and rivaroxaban should be minimized or avoided to reduce this risk 22

Extra Slides
CASPAR (DAPT in PAD Surgical Bypass) 851 patients with PAD undergoing surgical bypass randomized aspirin + placebo or clopidogrel + aspirin. DAPT had no benefit on the composite of index-graft occlusion or revascularization, above-ankle amputation of the affected limb, or death, HR 0. 98 (95% CI 0. 78 -1. 23, p=NS GUSTO bleeding was increased on aspirin + clopidogrel - HR 2. 84 (95% CI 1. 32 -6. 08) Study drug discontinuation (median follow up 1 year) was 21% on placebo and 25% on clopidogrel All-cause mortality HR 1. 44 (95% CI, 0. 77 -2. 68), CV death HR 1. 49 (95% CI, 0. 73 -3. 01) J Vasc Surg 2010; 52: 825 -3
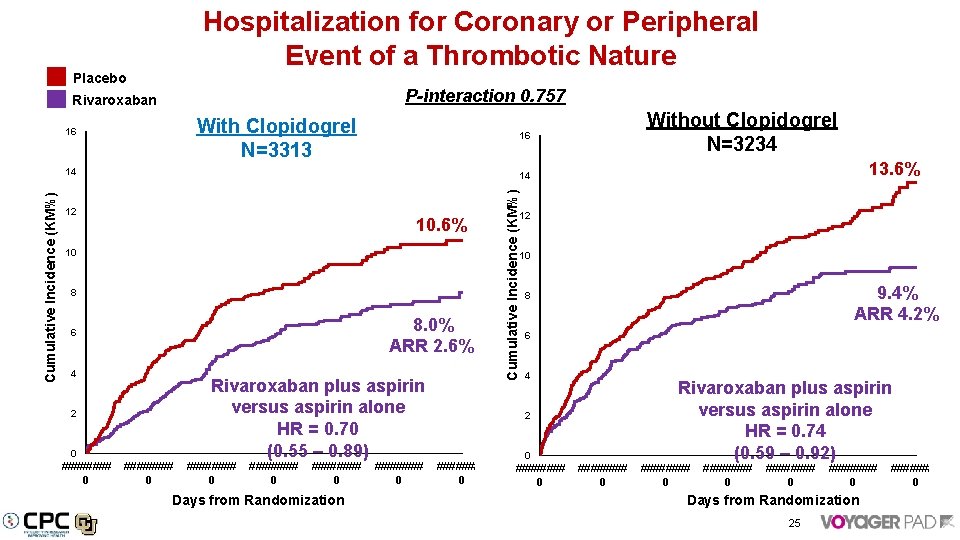
Hospitalization for Coronary or Peripheral Event of a Thrombotic Nature Placebo P-interaction 0. 757 Rivaroxaban With Clopidogrel N=3313 16 13. 6% 14 12 10. 6% 10 Cumulative Incidence (KM%) 14 12 10 8 8. 0% ARR 2. 6% 6 4 Rivaroxaban plus aspirin versus aspirin alone HR = 0. 70 (0. 55 – 0. 89) 2 0 #### 0 Without Clopidogrel N=3234 16 ######## 0 Days from Randomization #### 0 9. 4% ARR 4. 2% 8 6 4 2 #### 0 0 ######## 0 Rivaroxaban plus aspirin versus aspirin alone HR = 0. 74 (0. 59 – 0. 92) ######## 0 Days from Randomization 25 #### 0
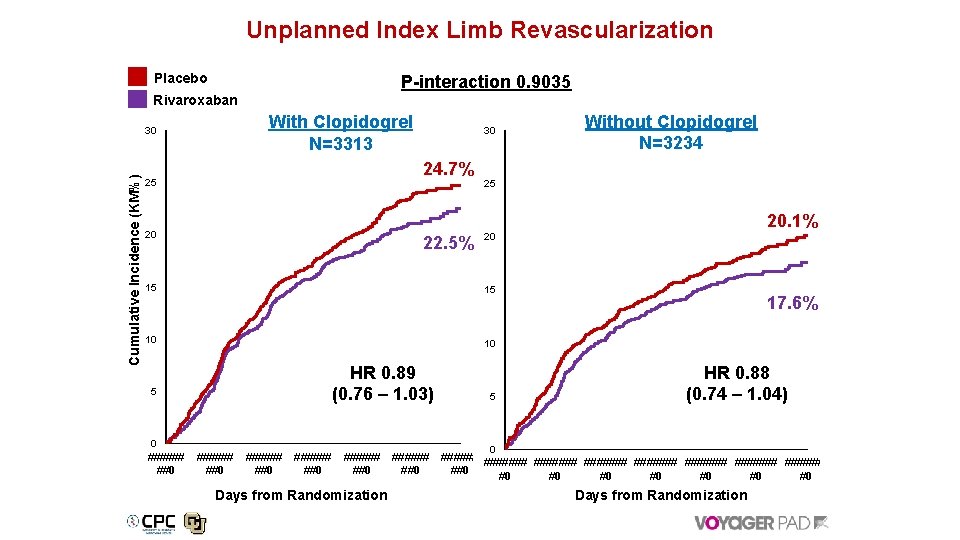
Unplanned Index Limb Revascularization Placebo P-interaction 0. 9035 Rivaroxaban With Clopidogrel N=3313 Cumulative Incidence (KM%) 30 30 24. 7% 25 25 20. 1% 20 22. 5% 15 20 15 10 17. 6% 10 HR 0. 89 (0. 76 – 1. 03) 5 0 ###### ##0 Without Clopidogrel N=3234 ###### ##0 Days from Randomization ###### ##0 5 HR 0. 88 (0. 74 – 1. 04) 0 ####### ####### ##0 #0 Days from Randomization
- Slides: 26