Volumetric Analysis Titrations Titrations Measures the amount of








- Slides: 14

Volumetric Analysis Titrations

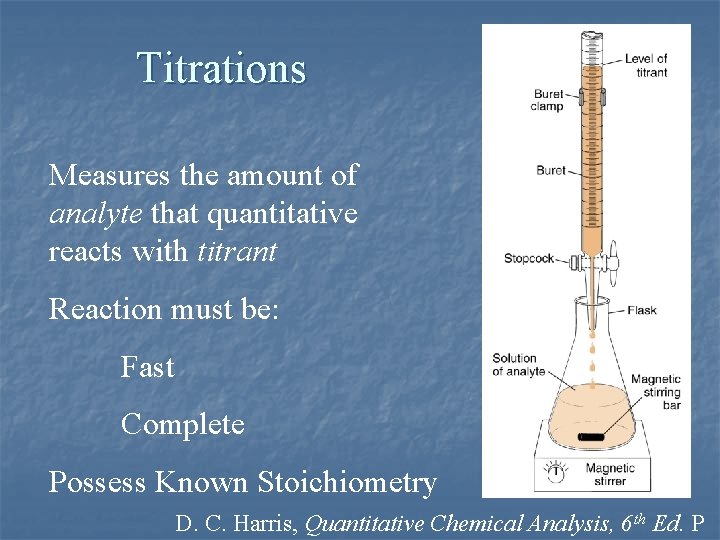
Titrations Measures the amount of analyte that quantitative reacts with titrant Reaction must be: Fast Complete Possess Known Stoichiometry D. C. Harris, Quantitative Chemical Analysis, 6 th Ed. P

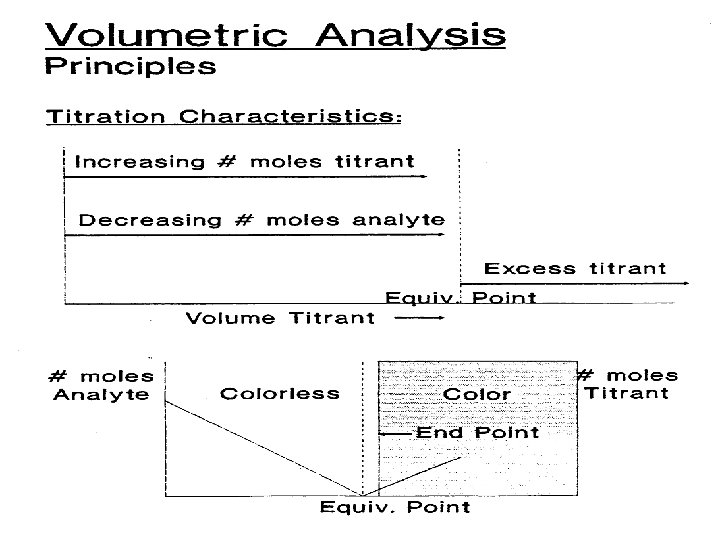
Volumetric Analysis Principles n n n “Titrimetry” – determination of analyte by reaction with measured amount of standard reagent “Standard Solution” (titrant) – reagent of known concentration “Titration” – slow addition of titrant to analyte solution from a volumetric vessel (buret) “Equivalence Point” – reached when amount of added titrant is chemically equivalent to amount of analyte present in the sample. “End Point” – the occurrence of an observable physical change indicating that the equivalence point is reached. Might differ from Eq. Pt. !

Volumetric Analysis - Principles How Do We Make Stoichiometric Calculations? n 1. 2. 3. 4. 5. 6. Titration- What are the requirements? Reaction must be stoichiometric Reaction should be rapid No side reactions Marked change in some property of the solution when reaction is complete Equivalence point Reaction should be quantitative

Standard Solutions: n stable, no change in concentration n react rapidly w/ analyte n react completely w/ analyte n react w/ known stoichiometry


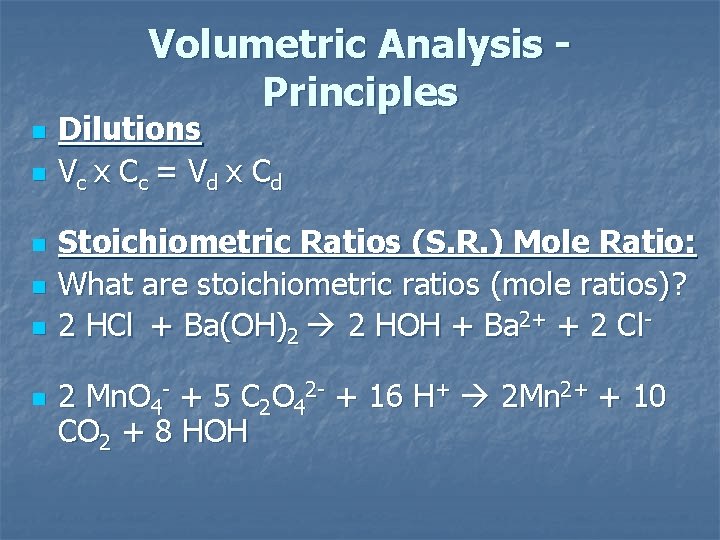
Volumetric Analysis Principles n n n Dilutions Vc x C c = V d x C d Stoichiometric Ratios (S. R. ) Mole Ratio: What are stoichiometric ratios (mole ratios)? 2 HCl + Ba(OH)2 2 HOH + Ba 2+ + 2 Cl 2 Mn. O 4 - + 5 C 2 O 42 - + 16 H+ 2 Mn 2+ + 10 CO 2 + 8 HOH

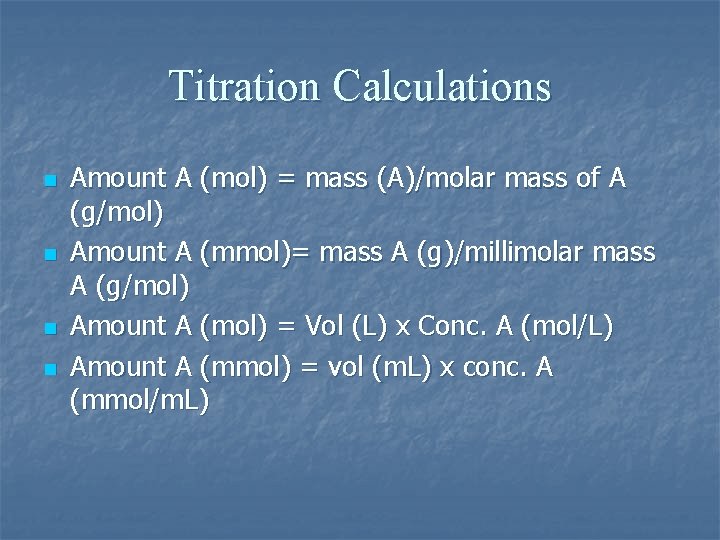
Titration Calculations n n Amount A (mol) = mass (A)/molar mass of A (g/mol) Amount A (mmol)= mass A (g)/millimolar mass A (g/mol) Amount A (mol) = Vol (L) x Conc. A (mol/L) Amount A (mmol) = vol (m. L) x conc. A (mmol/m. L)

n A 0. 8345 g sample of potassium hydrogen phthalate required 38. 31 m. L of 0. 1023 M Na. OH to reach the endpoint. What is the concentration (M) of Na. OH? n A 10. 00 m. L sample of an HCl solution to be used as an acid standard required 28. 45 m. L of the previously standardized Na. OH to reach the endpoint. What is the concentration of the HCl solution?

Molecular Weight Determination n A 0. 1046 g diprotic organic acid required 23. 47 m. L of 0. 1072 M standard KOH to reach the first endpoint. What is the molecular weight of the acid?

n % acidity = Mb Vb (A/B) Mwa x 100 weight (g) sample

Effect in the volume of the base consumed during titration n n 50 m. L dist. H 2 O instead of 20 m. L was added into the solution. Base buret was not rinsed with the solution of the base. The tip of the base buret was not completely filled. Some of the acid solution spilled during the titration.

Effect on % acidity n n n The tip of the buret was not filled with the solution before the initial reading was taken. Acid sample splashed out of the flask during titration. Molarity of the base solution used in the calculation was wrong, the true molarity being greater than the stated molarity.

end