Volumetric analysis Titration General chemistry The analysis of












- Slides: 28

Volumetric analysis. Titration. General chemistry.

• The analysis of a gas, liquid or solid sample or mixture to determine the precise percentage composition of the sample in terms of elements, radicals, or compounds.

• Quantitative analysis the aggregate of chemical, physicochemical methods of determining the quantitative ratios of constituents in the substance being analyzed. Together with qualitative analysis quantitative analysis is one of the major branches of analytical chemistry.

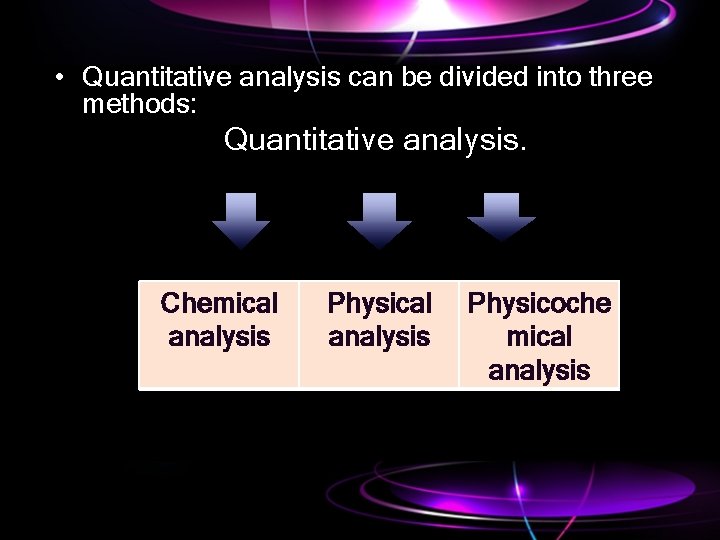
• Quantitative analysis can be divided into three methods: Quantitative analysis. Chemical analysis Physicoche mical analysis

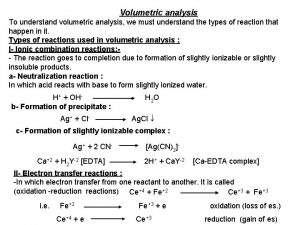
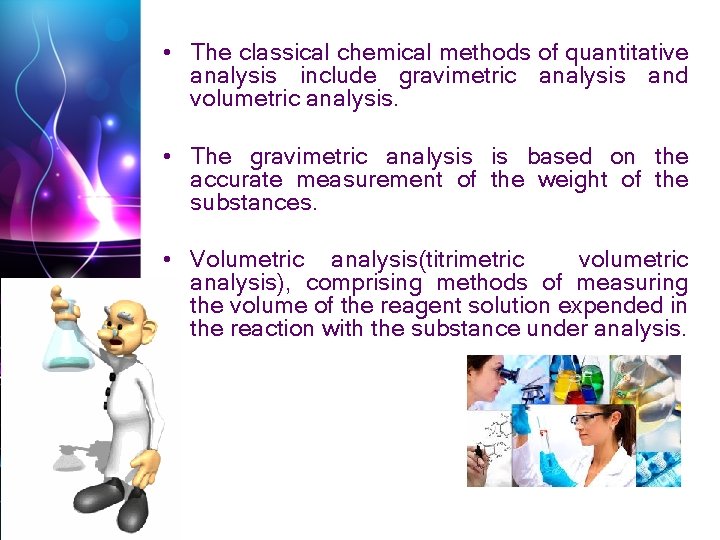
• The classical chemical methods of quantitative analysis include gravimetric analysis and volumetric analysis. • The gravimetric analysis is based on the accurate measurement of the weight of the substances. • Volumetric analysis(titrimetric volumetric analysis), comprising methods of measuring the volume of the reagent solution expended in the reaction with the substance under analysis.

• The physical and physicochemical (instrumental) methods are based on the measurement of optical, electrical, adsorption, catalytic, and other characteristics of the substances that are depend on their amount (concentration).

• These methods are usually divided into following groups: electrochemical methods(conductometry, polarography, potentiometry);

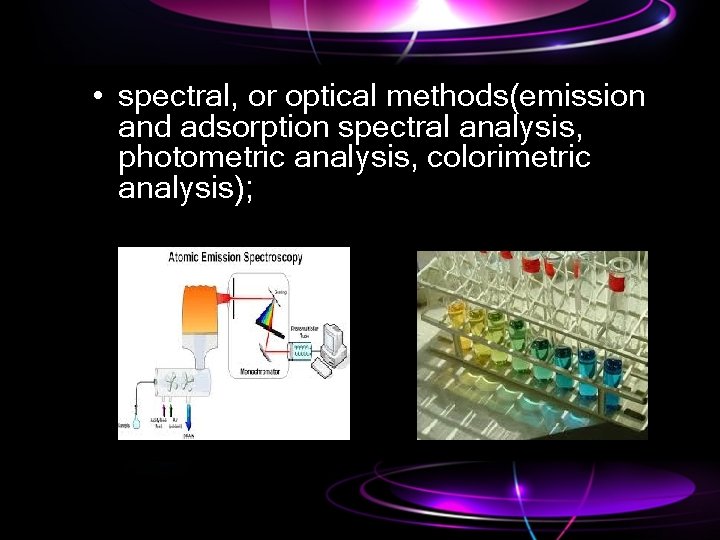
• spectral, or optical methods(emission and adsorption spectral analysis, photometric analysis, colorimetric analysis);

• chromatographic methods;

• X-ray phase analysis; radiometric methods and mass spectrometric methods.

Classification of Titrimetric Analysis methods •

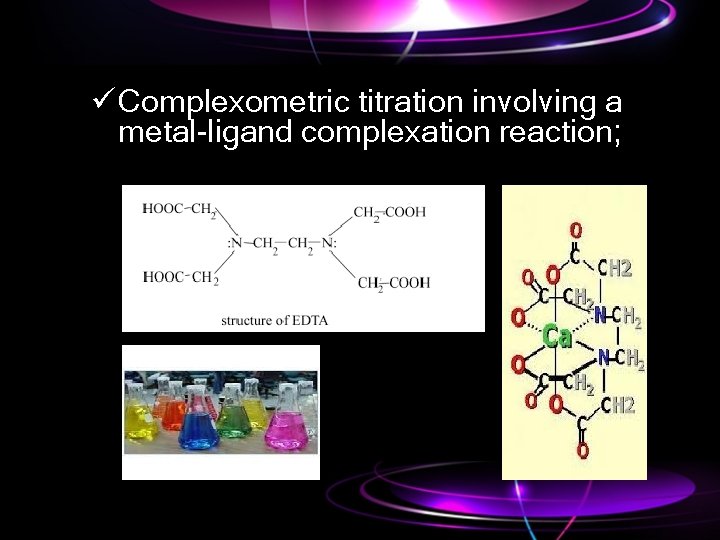
ü Complexometric titration involving a metal-ligand complexation reaction;

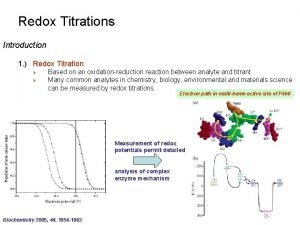
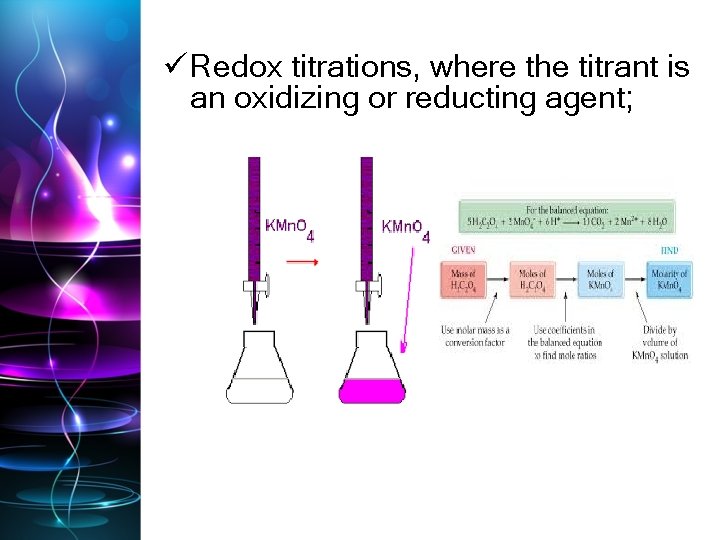
ü Redox titrations, where the titrant is an oxidizing or reducting agent;

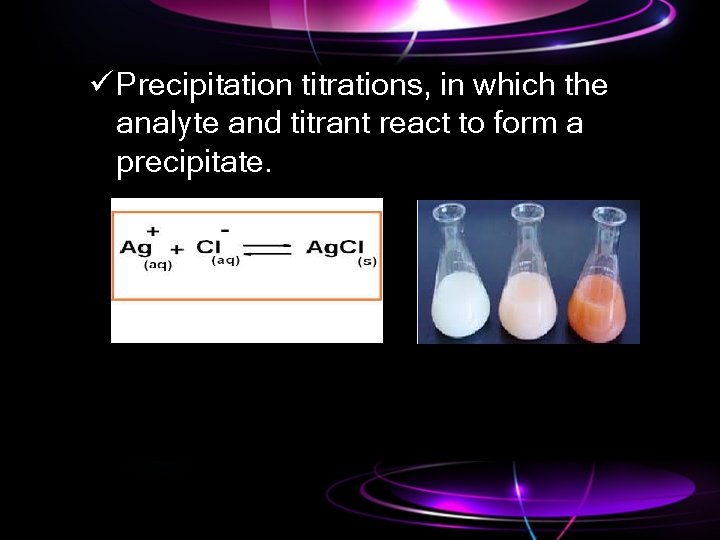
ü Precipitation titrations, in which the analyte and titrant react to form a precipitate.

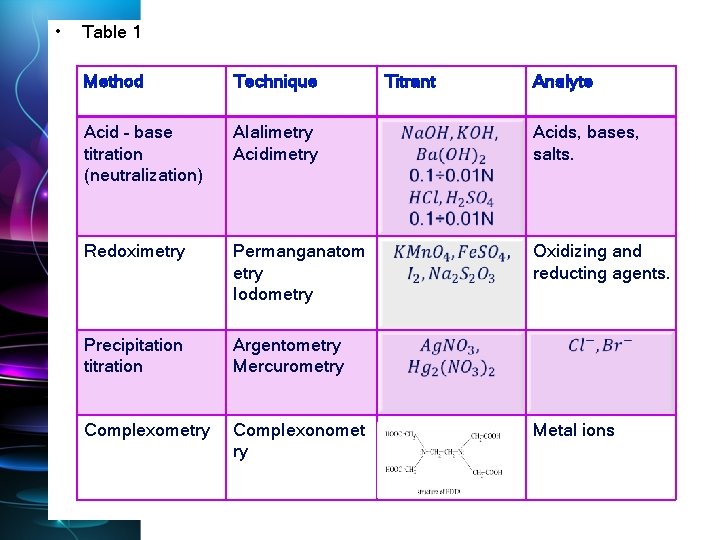
• Table 1 Method Technique Titrant Analyte Acid – base titration (neutralization) Alalimetry Acids, bases, salts. Redoximetry Permanganatom etry Iodometry Oxidizing and reducting agents. Precipitation titration Argentometry Mercurometry Complexonomet ry Metal ions

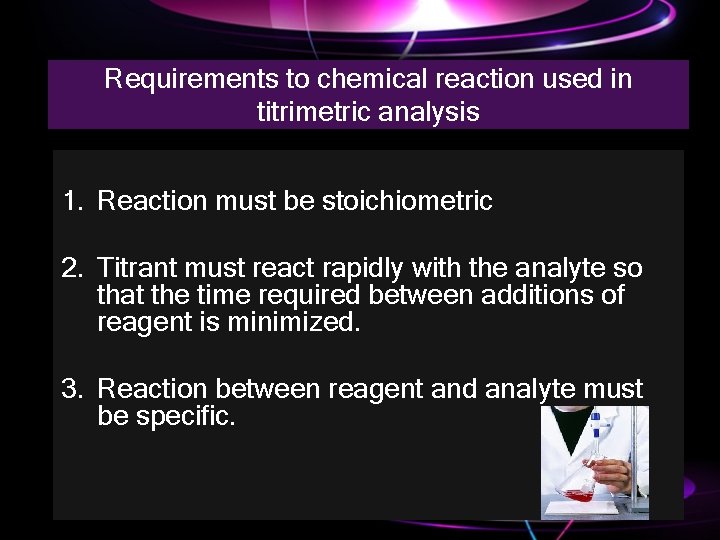
Requirements to chemical reaction used in titrimetric analysis 1. Reaction must be stoichiometric 2. Titrant must react rapidly with the analyte so that the time required between additions of reagent is minimized. 3. Reaction between reagent and analyte must be specific.

4. Titrant must react more or less completely with the analyte so that satisfactory and points are realized. 5. Undergo a selective reaction with the analyte that can be described by simple balanced equation. Equilibrium constant must have high value.

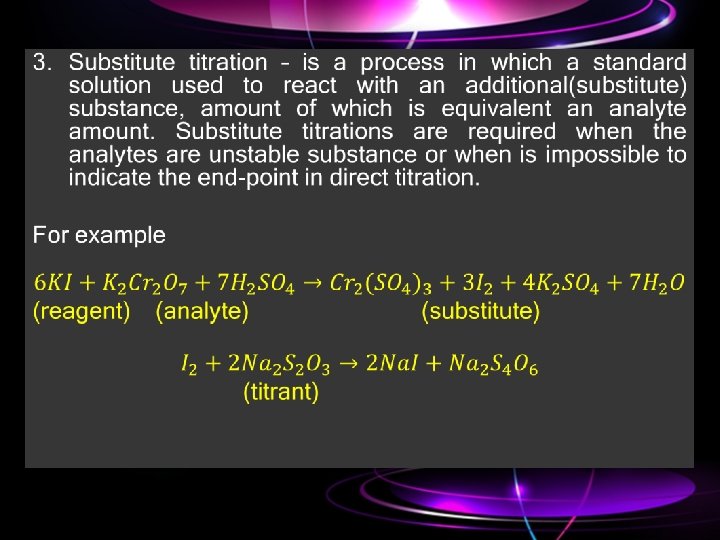
Types of titration. 1. Direct titration – titrant add to an analyte solution and react with determining substance;



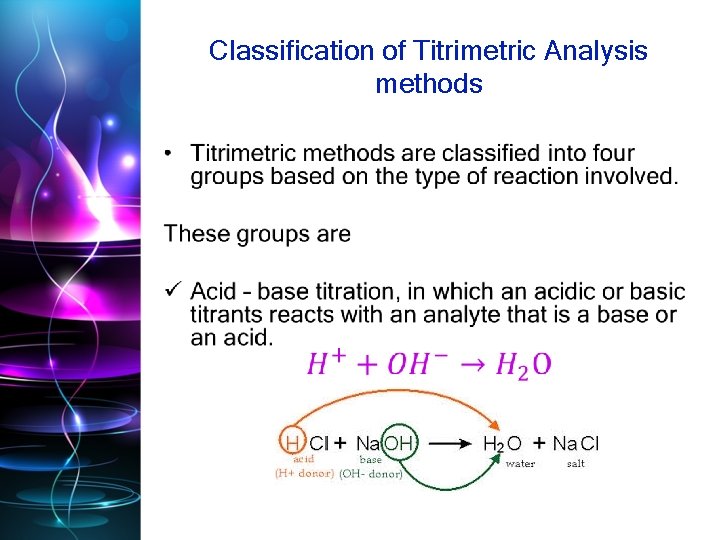
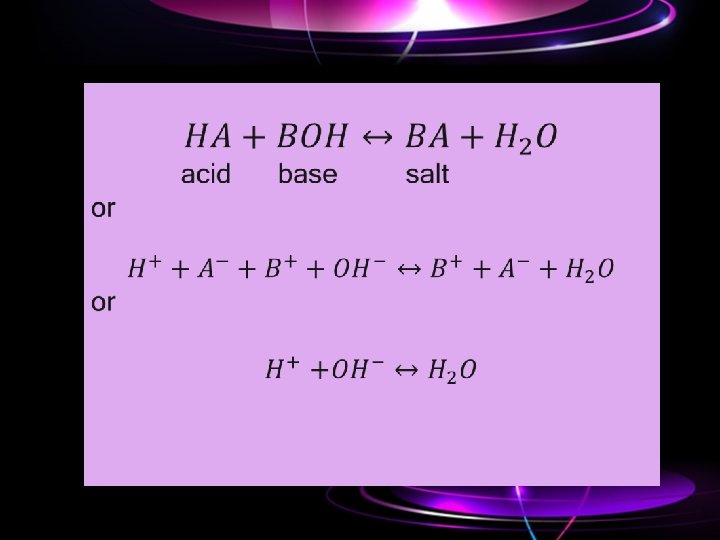
• Acid-base titrations: When the strength of an acid is determined with the help of a standard solution of base, it is known as acidimetry. Similarly, when the strength of a base (alkali) is determined with the help of a standard solution of an acid, it is known as alkalimetry. Both these titrations involve neutralization of an acid with an alkali. In these titrations H+ ions of the acid combine with OHions of the alkali to form ionized molecules of water.


• The end point in these titrations is determined by the use of organic dyes which are either weak acids or weak bases. These change their colors within a limited range of hydrogen ion concentrations, i. e. , p. H of the solution. Phenolphthalein is a suitable indicator in the titrations of strong alkalies (free from carbonate) against strong acids or weak acids. Methyl orange is used as an indicator in the titrations of strong acids against strong and weak alkalies.

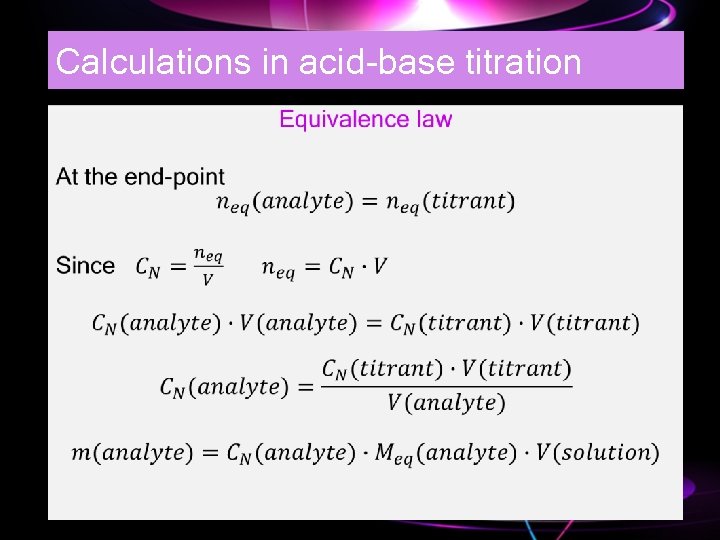
Calculations in acid-base titration •

Practical problem 1 •

Practical problem 2 •

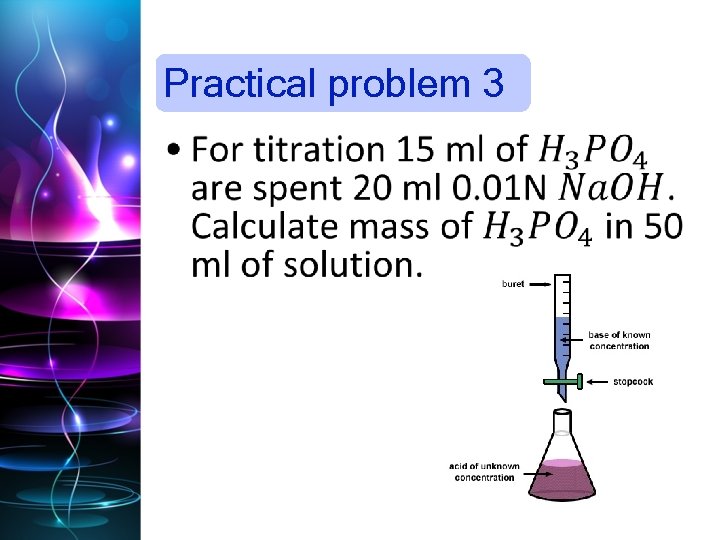
Practical problem 3 •

Practical problem 4 •
 Titration definition
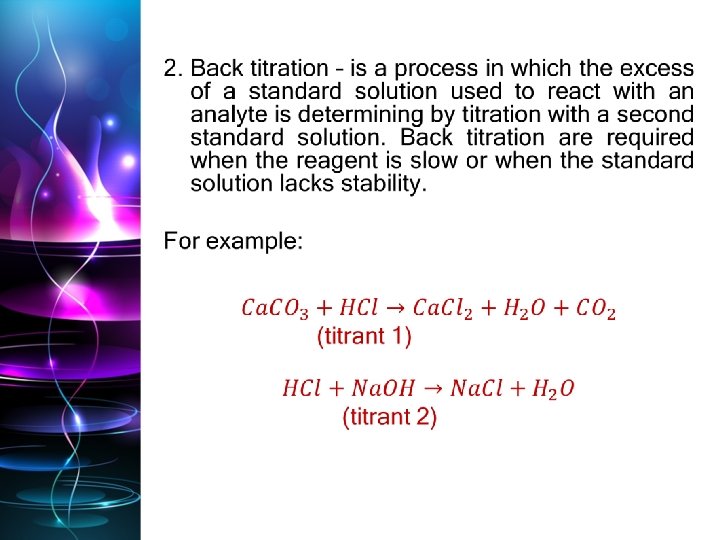
Titration definition Back titrations
Back titrations Define volumetric analysis
Define volumetric analysis Redox titration worksheet
Redox titration worksheet Redox titration definition
Redox titration definition Gravimetric analysis problems
Gravimetric analysis problems Complexometric titration definition
Complexometric titration definition Volumetric analysis equipment
Volumetric analysis equipment Introduction of gravimetric analysis
Introduction of gravimetric analysis Difference between gravimetric and volumetric analysis
Difference between gravimetric and volumetric analysis Iodine and sodium thiosulfate
Iodine and sodium thiosulfate Precipitation titration
Precipitation titration Titration picture
Titration picture Ma*va=mb*vb
Ma*va=mb*vb General chemistry with qualitative analysis
General chemistry with qualitative analysis Volumetric efficiency
Volumetric efficiency Compression ratio definition in engine
Compression ratio definition in engine Volumetric glassware and routine glassware
Volumetric glassware and routine glassware Graduated glassware examples
Graduated glassware examples Volumetric efficiency
Volumetric efficiency Florence flasks
Florence flasks Volumetric vs mohr pipette
Volumetric vs mohr pipette Volumetric vs mohr pipette
Volumetric vs mohr pipette Volumetric properties of pure fluids
Volumetric properties of pure fluids Osmometric thirst and volumetric thirst
Osmometric thirst and volumetric thirst Volumetric
Volumetric Upbp
Upbp Volumetric efficiency formula
Volumetric efficiency formula Efficiency and effectiveness examples
Efficiency and effectiveness examples