Volumetric Analysis Titration An introduction The Big Picture
Volumetric Analysis: Titration An introduction

The Big Picture Use a solution of known concentration (the standard solution) to determine the concentration of an unknown solution

What we need • The chemical equation • A way to measure amounts of solution added – Fixed and variable • A way to determine that the titration is complete • Reaction should be fast
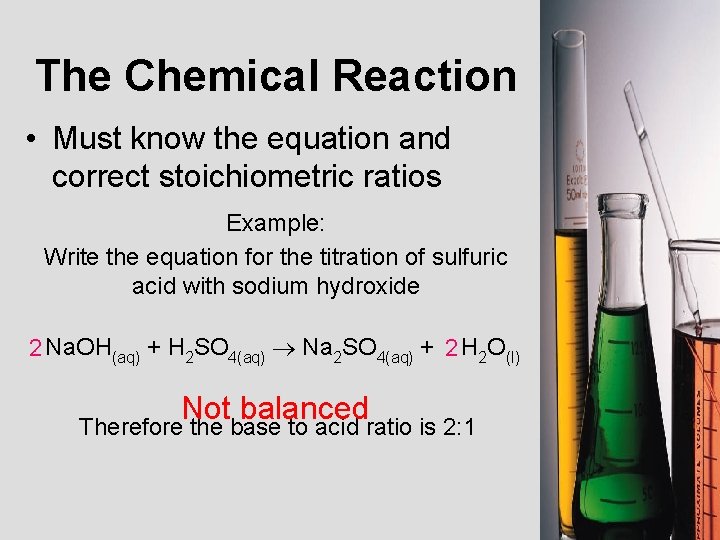
The Chemical Reaction • Must know the equation and correct stoichiometric ratios Example: Write the equation for the titration of sulfuric acid with sodium hydroxide 2 Na. OH(aq) + H 2 SO 4(aq) Na 2 SO 4(aq) + 2 H 2 O(l) Not balanced Therefore the base to acid ratio is 2: 1
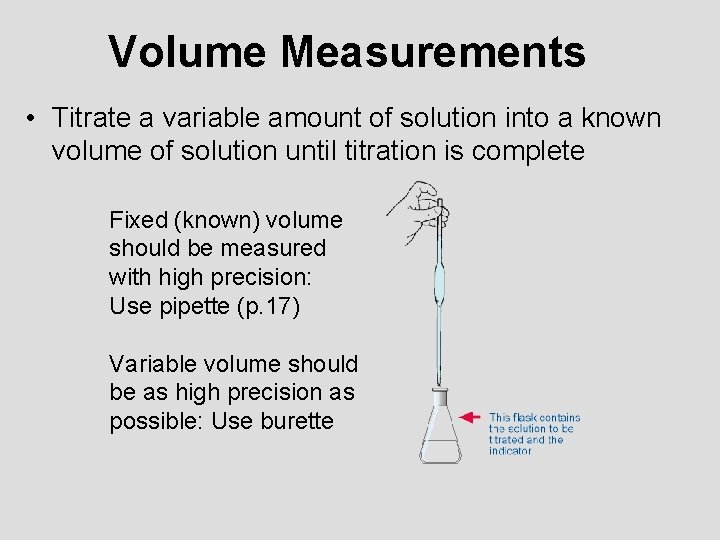
Volume Measurements • Titrate a variable amount of solution into a known volume of solution until titration is complete Fixed (known) volume should be measured with high precision: Use pipette (p. 17) Variable volume should be as high precision as possible: Use burette
How to determine titration is complete • Usually an indicator is used Terms End point: when indicator changes color Equivalence point: when a stoichiometrically equivalent amount of titrant has been added to solution

More on indicators Phenolphthalein is a very common acid-base indicator Colorless in acid Pink in base

Example 25. 00 m. L of 0. 460 M sulfuric acid is titrated with Na. OH, requiring 28. 45 m. L to reach the endpoint. What is the Na. OH concentration?

Example We place 25. 00 m. L of acid and a couple drops of the indicator into the flask and titrate with base until the solution turns slightly pink. 2 Na. OH(aq) + H 2 SO 4(aq) Na 2 SO 4(aq) + 2 H 2 O(l)

Example 25. 00 m. L of 0. 460 M sulfuric acid is titrated with Na. OH, requiring 28. 45 m. L to reach the endpoint. What is the Na. OH concentration?

Example Moles of acid Moles of base Concentration of base

Some Titration Techniques • Start with burette at or beyond 0. 00 m. L – It doesn’t matter, just record it! – Don’t waste time getting exactly 0. 00 m. L • Titrate to the ½ drop • Add extra water if needed – How does this affect calculations?

Some Titration Techniques • Stir! • Place on white paper • Palest and most reproducible pink possible
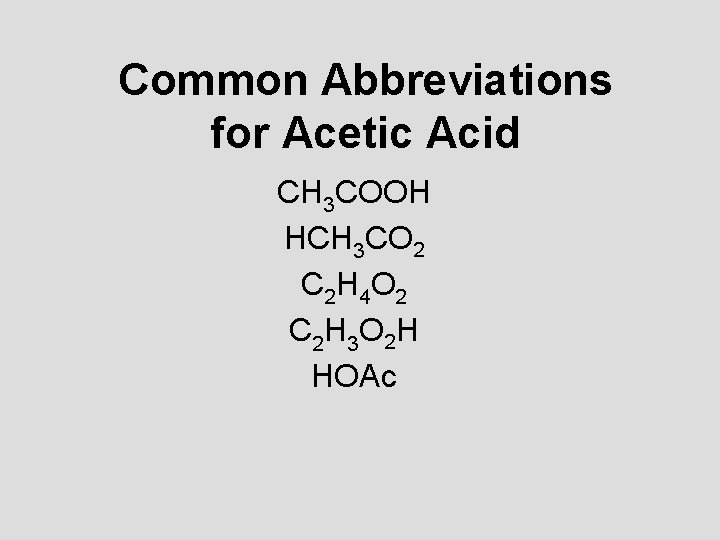
Common Abbreviations for Acetic Acid CH 3 COOH HCH 3 CO 2 C 2 H 4 O 2 C 2 H 3 O 2 H HOAc
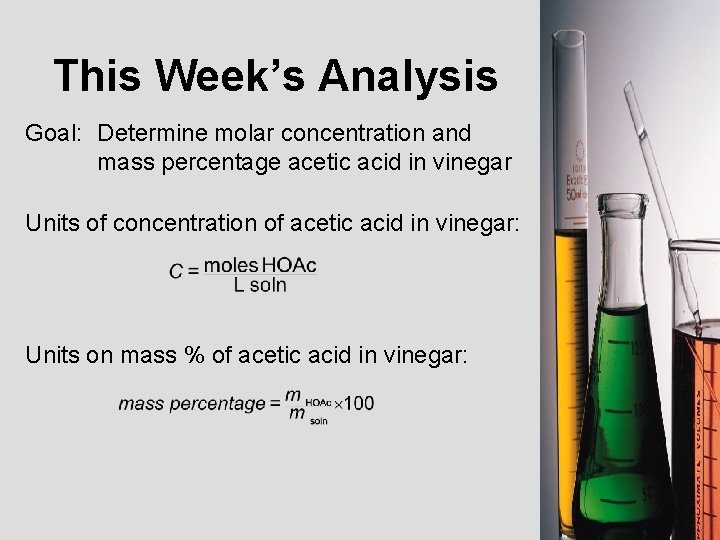
This Week’s Analysis Goal: Determine molar concentration and mass percentage acetic acid in vinegar Units of concentration of acetic acid in vinegar: Units on mass % of acetic acid in vinegar:
This Week’s Analysis • Oxalic acid as a primary standard – Why? • One oxalic acid solution per 2 groups • Note that oxalic acid is a hydrate – How does this affect you? • 10 m. L pipettes • 250 m. L volumetric flasks

Procedure Proposal How you will prepare your oxalic acid solution (~0. 25 M) How you will determine Na. OH concentration including calculations and chemical reactions How you will determine the concentration of the acetic acid in vinegar (and how many trials) Road map of calculations for calculating C and mass % acetic acid from your data Don’t even think of using C 1 V 1=C 2 V 2

This investigation, Author 3: Introduction and Conclusion 1: Discussion 2: Data/Results and Experimental This investigation, Author A: Introduction, Conclusion, Data/Results B: Discussion and Experimental
- Slides: 18