Volumetric Analysis Precipitation Titrations Mohrs Method Prepared at






- Slides: 11

Volumetric Analysis Precipitation Titrations (Mohr's Method) Prepared at May 2015 to 1 st stage in college of pharmacy / Hawler Medical University

Precipitation Titrations (Mohr's Method) Titrations in which precipitates are formed are called precipitation titrations. The most frequent application of this type of titration uses silver ion to determine chloride. Therefore, these titrations are called argentometric titrations. In analytical chemistry , argentometry is a type of titration involving • the silver(I) ion. Typically, it is used to determine the amount of chloride present in a sample. The sample solution is titrated against a solution of silver nitrate of known concentration. Chloride ions react with silver(I) ions to give the insoluble silver chloride: Ag+ (aq) + Cl- (aq) → Ag. Cl (s) • 2

According to the indicator used, three methods can be described. 1. Mohr's method : Chromate is the indicator. 2. Fajans method makes use of adsorption indicators. Both methods are direct methods. 3. Volhard method The third method is an indirect method where an excess silver is added to chloride unknown and the remaining silver is back-titrated with a standard thiocyanate solution in presence of Fe(III) as an indicator 3

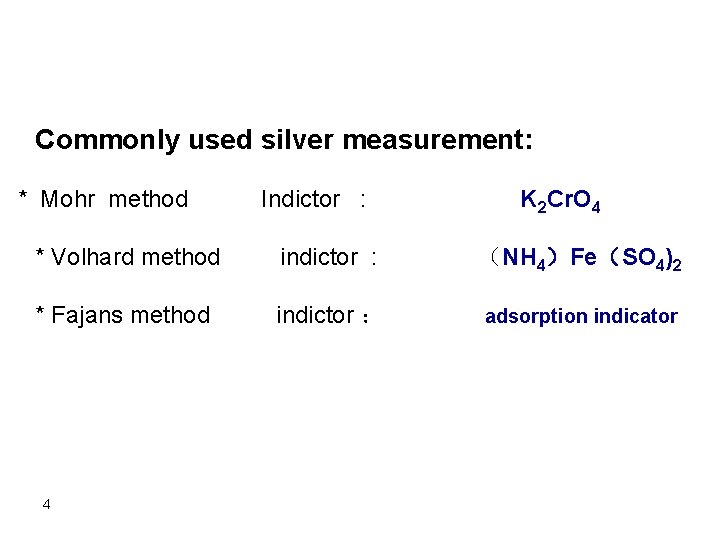
Commonly used silver measurement: * Mohr method Indictor : K 2 Cr. O 4 * Volhard method indictor : (NH 4)Fe(SO 4)2 * Fajans method indictor : adsorption indicator 4

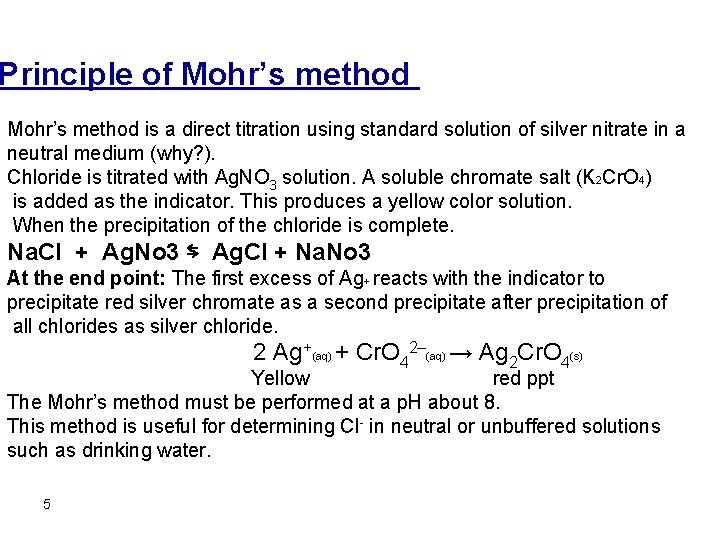
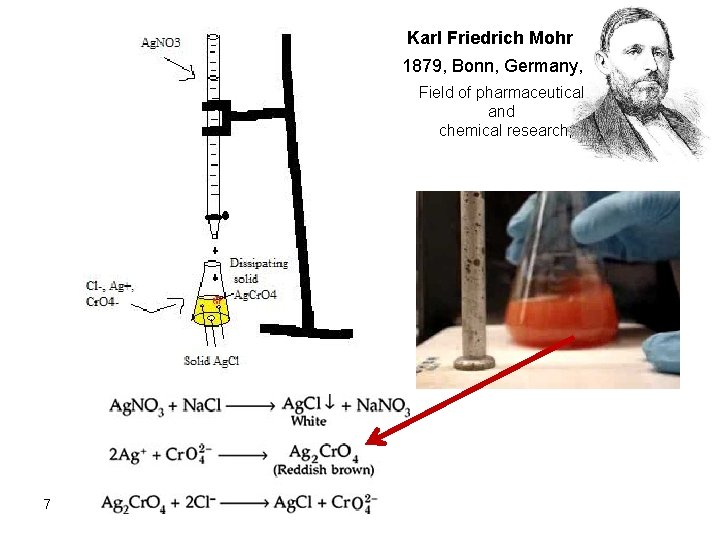
Principle of Mohr’s method is a direct titration using standard solution of silver nitrate in a neutral medium (why? ). Chloride is titrated with Ag. NO 3 solution. A soluble chromate salt (K 2 Cr. O 4) is added as the indicator. This produces a yellow color solution. When the precipitation of the chloride is complete. Na. Cl + Ag. No 3 ⇆ Ag. Cl + Na. No 3 At the end point: The first excess of Ag+ reacts with the indicator to precipitate red silver chromate as a second precipitate after precipitation of all chlorides as silver chloride. 2 Ag +(aq) + Cr. O 42–(aq) → Ag 2 Cr. O 4(s) Yellow red ppt The Mohr’s method must be performed at a p. H about 8. This method is useful for determining Cl- in neutral or unbuffered solutions such as drinking water. 5

Procedure: 1. Pipette 10 ml of sodium chloride solution (Na. Cl) into stoppered conical flask. 3. Add 1 ml of 2% neutral potassium chromate indicator. 4. Titrate with 0. 05 N Ag. NO 3 solution, swirling the liquid constantly, until the red colour-formed by addition of each drop of Ag. NO 3 solution begins to disappear more slowly; this is an indication that most of chloride has been precipitated and that the end point is near. 5. Continue the titration, drop wise, until a faint, but distinct, brick red color is formed and does not disappear on vigorous shaking. End point: brick red color 6

Karl Friedrich Mohr 1879, Bonn, Germany, Field of pharmaceutical and chemical research; 7

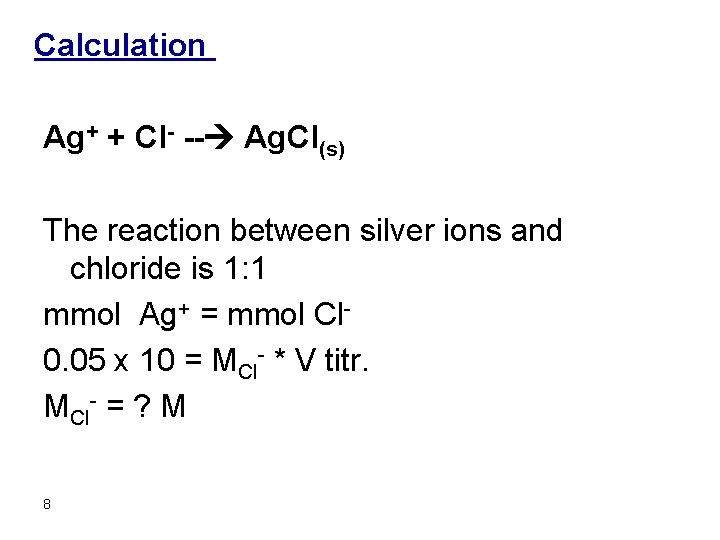
Calculation Ag+ + Cl- -- Ag. Cl(s) The reaction between silver ions and chloride is 1: 1 mmol Ag+ = mmol Cl 0. 05 x 10 = MCl- * V titr. MCl- = ? M 8

Home Work? 1. Adsorption indicators. 2. Mohr’s method is a direct titration using standard solution of silver nitrate in a neutral medium (why? ). 3. Definition of Fajans method • 4. Definition Volhard method • 9

T HANKS F O R L I S T E NI NG I T S T I ME F O R Q U E S T I O N S 10

In this method, neutral medium should be used since, in alkaline solutions, silver will react with the hydroxide ions forming Ag. OH. In acidic solutions, chromate will be converted to dichromate. Therefore, the p. H of solution should be kept at about 7. There is always some error in this method because a dilute chromate solution is used due to the intense color of the indicator. This will require additional amount of Ag+ for the Ag 2 Cr. O 4 to form. 11