Volume vs Temperature of a Gas Problem What

Volume vs. Temperature of a Gas Problem: What is the relationship between the volume and temperature of a gas? Hypothesis: As the temperature of a gas increases its’ volume ______ because … No procedure No Diagram
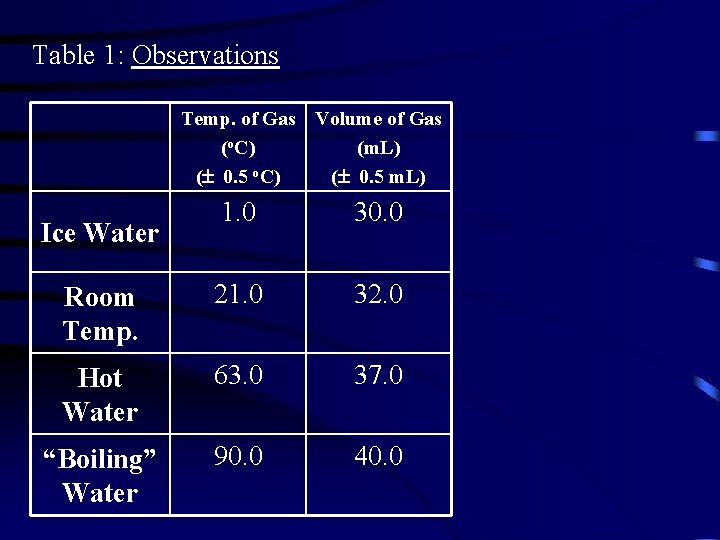
Table 1: Observations Temp. of Gas Volume of Gas (o. C) (m. L) ( 0. 5 o. C) ( 0. 5 m. L) 1. 0 30. 0 Room Temp. 21. 0 32. 0 Hot Water 63. 0 37. 0 “Boiling” Water 90. 0 40. 0 Ice Water

Analysis: 1. 2. 3. 4. 5. Graph your results. Extrapolate the graph to zero volume. Note/record the temperature when the volume is 0 m. L. This is the lowest possible temperature that can exist in nature. Indicate on your graph the accepted value for “Absolute Zero” = -273 o. C On the top left hand corner of your graph calculate the: 1. Experimental Error E = O - A 2. % Error = E X 100 A 6. Complete the table on the next slide. Conclusions: 1. 2. This is a ________ (direct/indirect) relationship. The data ________ (supports/does not support) the hypothesis.
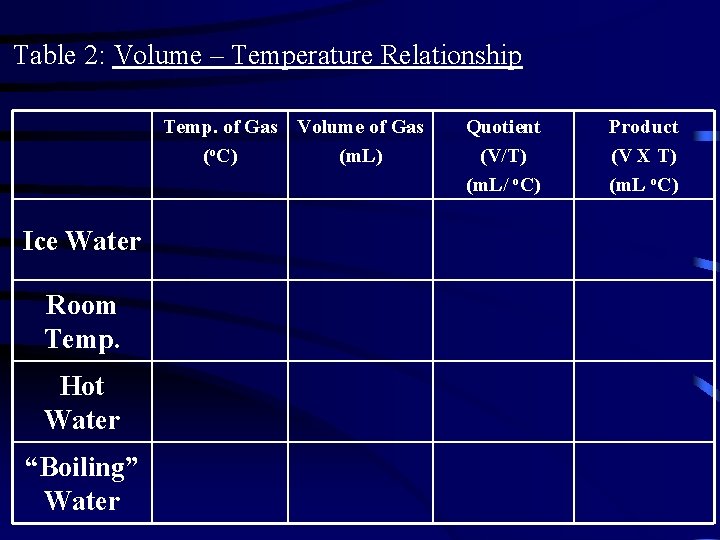
Table 2: Volume – Temperature Relationship Temp. of Gas Volume of Gas (o. C) (m. L) Ice Water Room Temp. Hot Water “Boiling” Water Quotient (V/T) (m. L/ o. C) Product (V X T) (m. L o. C)
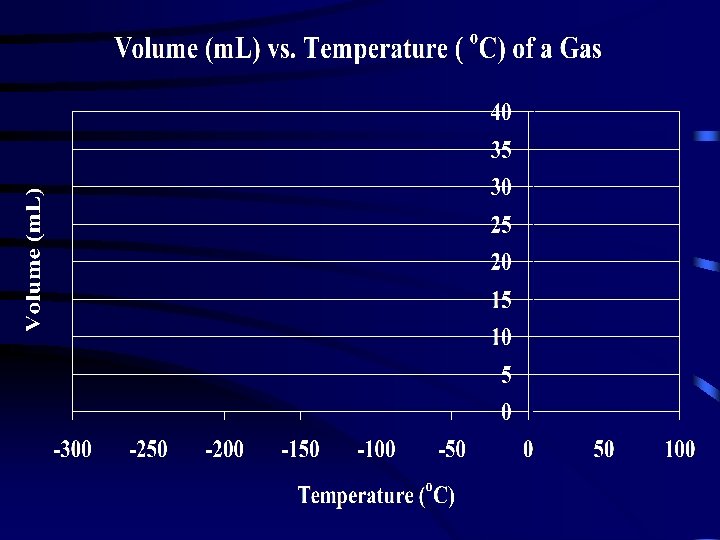
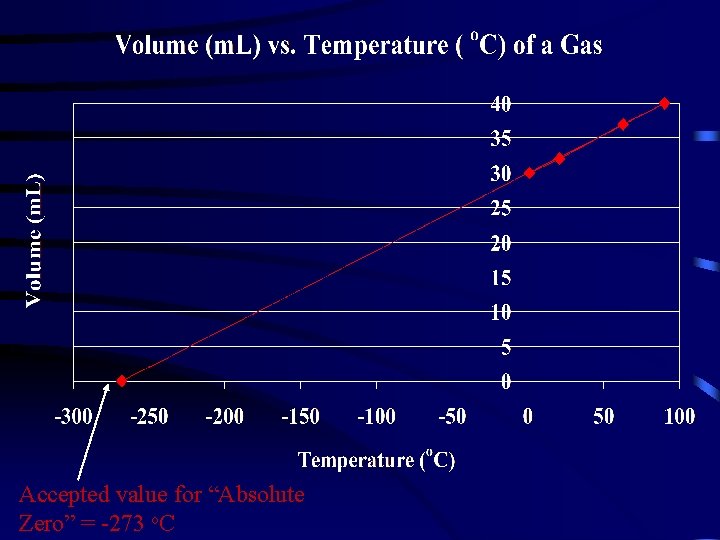
Accepted value for “Absolute Zero” = -273 o. C
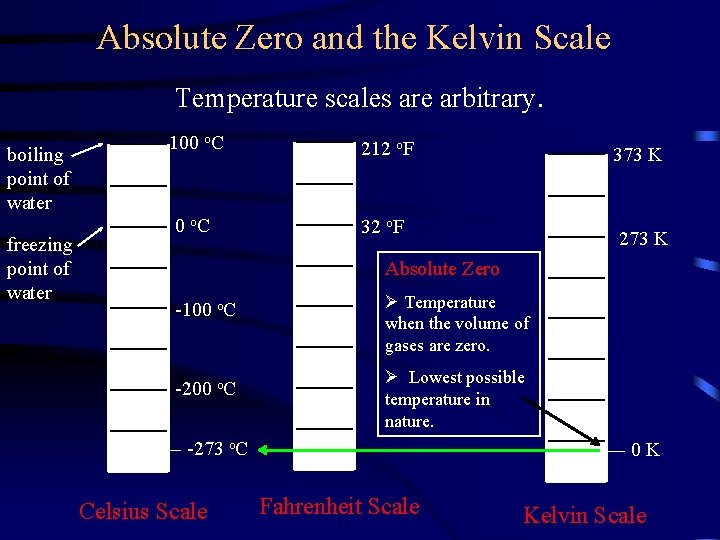
Absolute Zero and the Kelvin Scale Temperature scales are arbitrary. boiling point of water freezing point of water 100 o. C 212 o. F 0 o. C 32 o. F 373 K 273 K Absolute Zero -100 o. C -200 o. C Ø Temperature when the volume of gases are zero. Ø Lowest possible temperature in nature. -273 o. C Celsius Scale 0 K Fahrenheit Scale Kelvin Scale

Absolute Zero and the Kelvin Scale Absolute Zero ØLowest possible temperature in nature ØEquivalent to – 273 o. C Converting from degrees Celsius to Kelvin (absolute scale) Temperature K = Temperature o. C + 273 Notes: • A degree Celsius is equal in size to a degree Kelvin • Do not use the degree symbol (o) when writing temperature in Kelvin. (i. e. write 50 K not 50 o. K)

Ch. 2 p. 23 # 28 – 32

Which of the following expressions best describes Charles’ Law? q V X T = constant q V + T = constant q V T = constant q V – T = constant
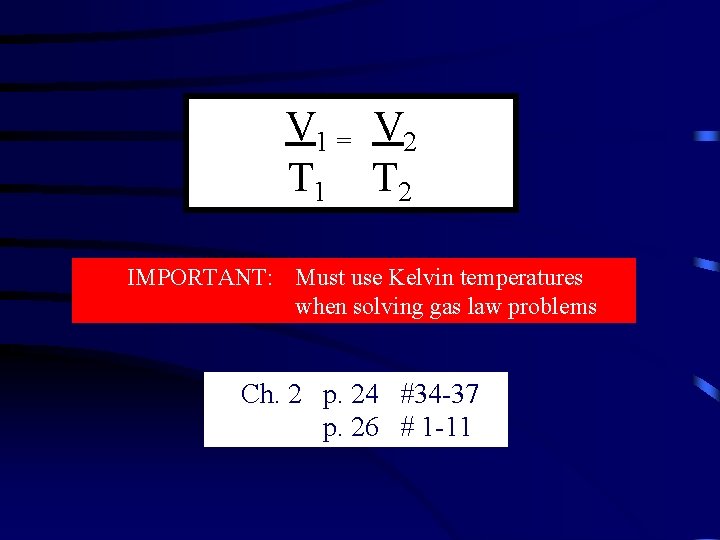
V 1 = V 2 T 1 T 2 IMPORTANT: Must use Kelvin temperatures when solving gas law problems Ch. 2 p. 24 #34 -37 p. 26 # 1 -11
- Slides: 11