VOLTMETER SLIDE ONE OVERVIEW ANIMATION 2 e 2
- Slides: 4

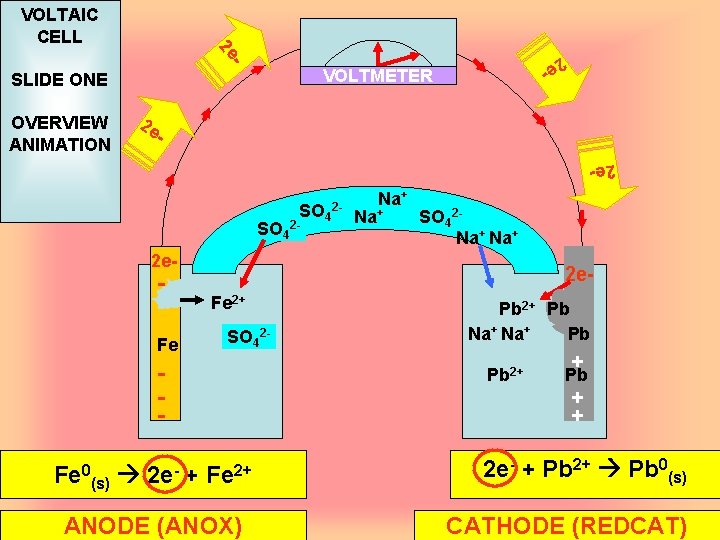
- VOLTMETER SLIDE ONE OVERVIEW ANIMATION 2 e - 2 e VOLTAIC CELL 2 e - 2 e. SO 4 2 - 2 - Na+ SO 42 Na+ 2 e- - Fe Fe 2 e. Fe 2+ SO 42 - Fe 0(s) 2 e- + Fe 2+ ANODE (ANOX) Pb 2+ Pb Na+ Pb Pb 2+ + Pb 2 e- + Pb 2+ Pb 0(s) CATHODE (REDCAT)

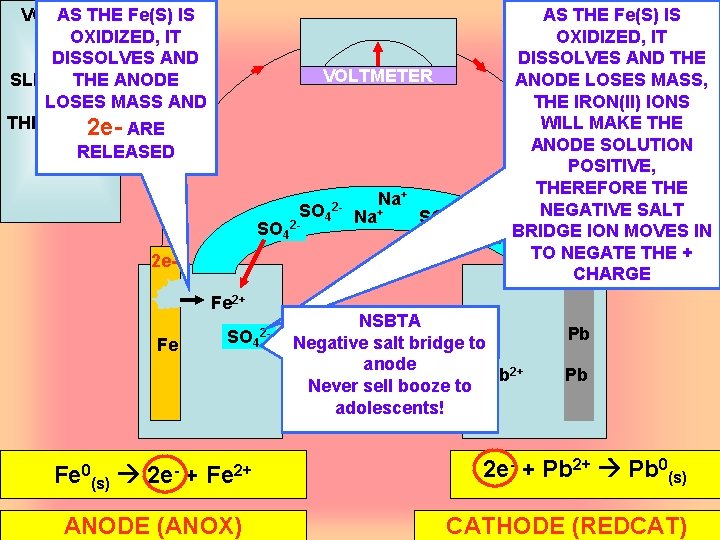
VOLTAIC AS THE Fe(S) IS CELL OXIDIZED, IT DISSOLVES AND SLIDE TWO THE ANODE LOSES MASS AND THE ANODE 2 e- ARE AS THE Fe(S) IS OXIDIZED, IT DISSOLVES AND THE VOLTMETER ANODE LOSES MASS, THE IRON(II) IONS WILL MAKE THE ANODE SOLUTION POSITIVE, THEREFORE THE + Na NEGATIVE SALT SO 42 - Na+ 2 SO 42 ION MOVES IN + Na. BRIDGE TO NEGATE THE + CHARGE RELEASED 2 e. Fe Fe Fe 2+ SO 4 Fe 0(s) 2 e- + Fe 2+ ANODE (ANOX) 2 - NSBTA Negative salt bridge to anode Pb 2+ Never sell booze to adolescents! Pb Pb 2 e- + Pb 2+ Pb 0(s) CATHODE (REDCAT)

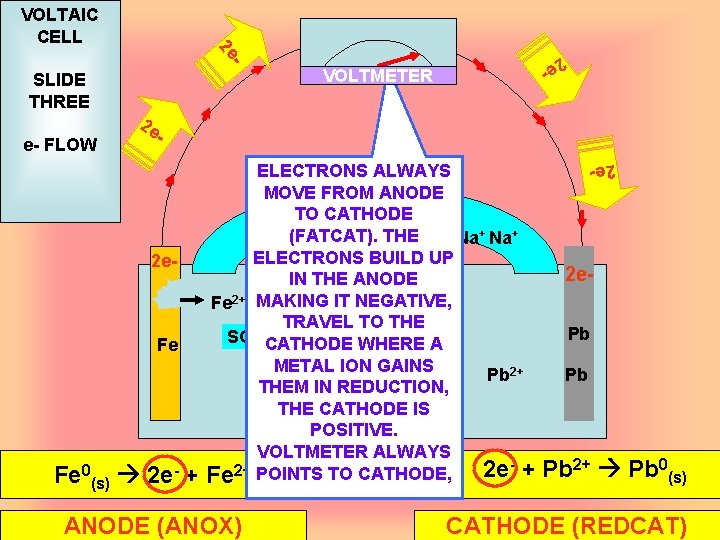
VOLTMETER 2 e - 2 e. Fe Fe Fe 0(s) 2 e- + ELECTRONS ALWAYS MOVE FROM Na ANODE + 2 SOCATHODE TO 4 Na+ SO 42(FATCAT). THE Na+ ELECTRONS BUILD UP IN THE ANODE Fe 2+ MAKING IT NEGATIVE, TRAVEL TO THE SO 42 -CATHODE WHERE A METAL ION GAINS Pb 2+ THEM IN REDUCTION, THE CATHODE IS POSITIVE. VOLTMETER ALWAYS Fe 2+ POINTS TO CATHODE, 2 e + ANODE (ANOX) 2 e- e- FLOW - SLIDE THREE 2 e - 2 e VOLTAIC CELL 2 e. Pb Pb Pb 2+ Pb 0(s) CATHODE (REDCAT)

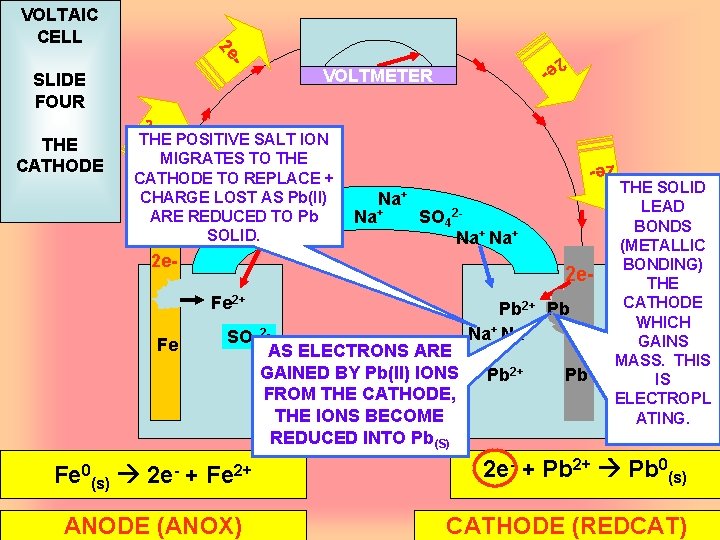
VOLTMETER 2 e - THE POSITIVE SALT ION MIGRATES TO THE CATHODE TO REPLACE + CHARGE LOST AS Pb(II) Na+ 2 SO 4 Na+ ARE REDUCED TO 2 -Pb SO 42 SOLID. SO 4 Na+ 2 e. Fe Fe 2 e- THE CATHODE - SLIDE FOUR 2 e - 2 e VOLTAIC CELL 2 e. Fe 2+ SO 42 AS ELECTRONS ARE GAINED BY Pb(II) IONS FROM THE CATHODE, THE IONS BECOME REDUCED INTO Pb(S) Fe 0(s) 2 e- + Fe 2+ ANODE (ANOX) Pb 2+ Pb Na+ Pb Pb 2+ Pb THE SOLID LEAD BONDS (METALLIC BONDING) THE CATHODE WHICH GAINS MASS. THIS IS ELECTROPL ATING. 2 e- + Pb 2+ Pb 0(s) CATHODE (REDCAT)