Voltammetry and Polarography Lecture 5 1 Differential Pulse






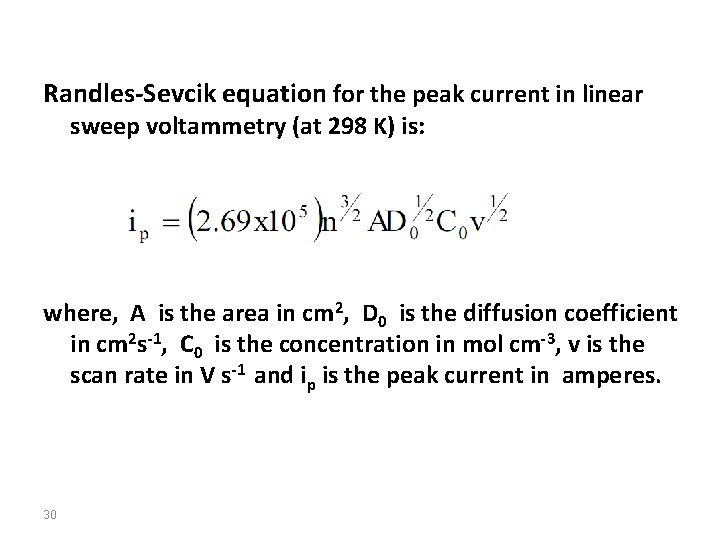

- Slides: 37

Voltammetry and Polarography Lecture 5 1

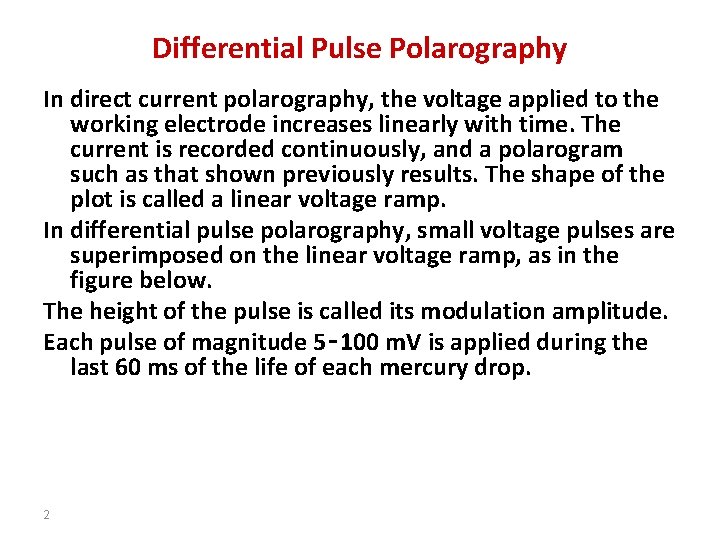
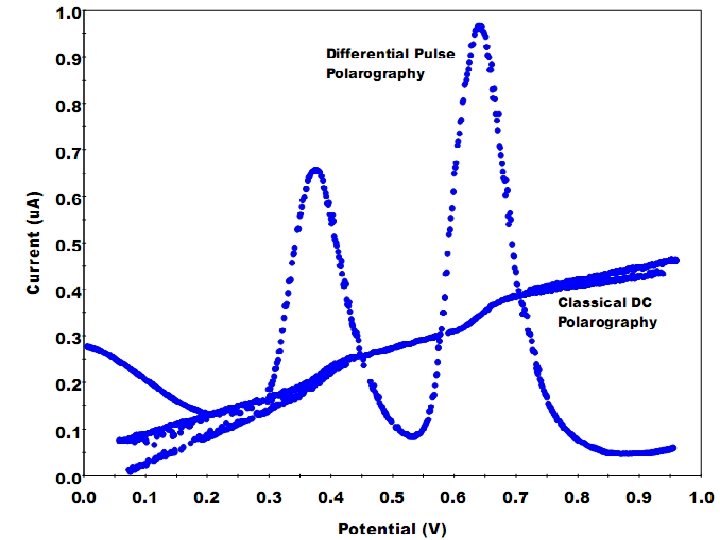
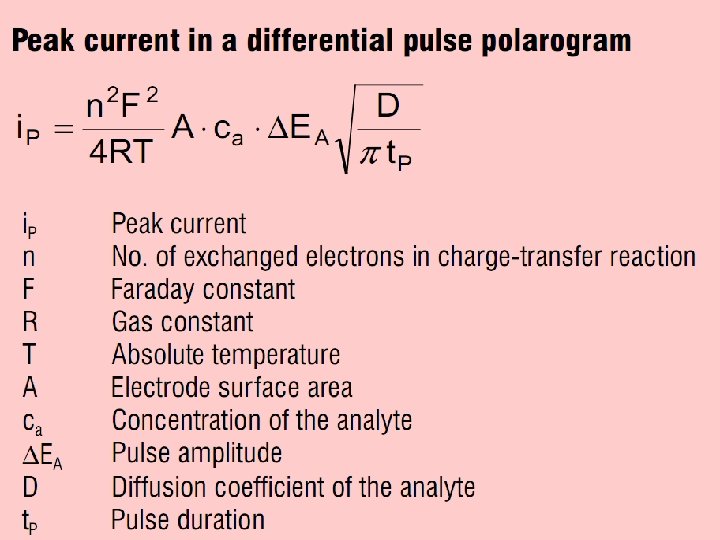
Differential Pulse Polarography In direct current polarography, the voltage applied to the working electrode increases linearly with time. The current is recorded continuously, and a polarogram such as that shown previously results. The shape of the plot is called a linear voltage ramp. In differential pulse polarography, small voltage pulses are superimposed on the linear voltage ramp, as in the figure below. The height of the pulse is called its modulation amplitude. Each pulse of magnitude 5‑ 100 m. V is applied during the last 60 ms of the life of each mercury drop. 2

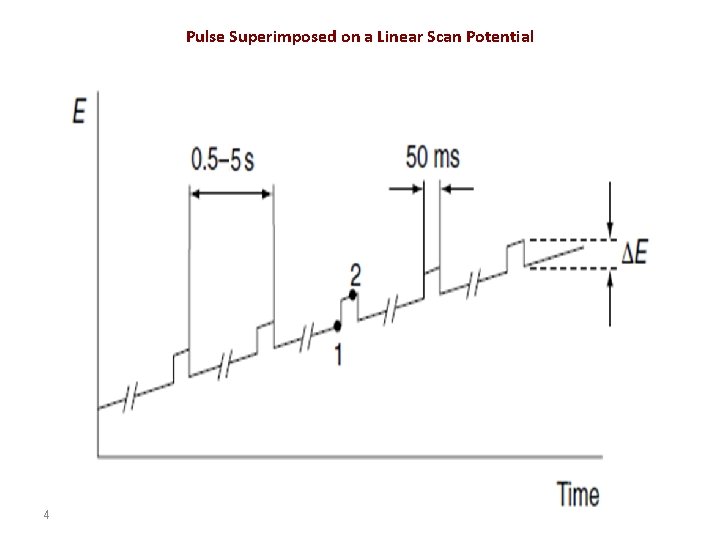
• The drop is then mechanically dislodged. • The current is not measured continuously. Rather, it is measured once before the pulse and again for the last 17 ms of the pulse. • The polarograph subtracts the first current from the second and plots this difference versus the applied potential (measured just before the voltage pulse). • The resulting differential pulse polarogram is nearly the derivative of a direct current polarogram. 3

Pulse Superimposed on a Linear Scan Potential 4

5

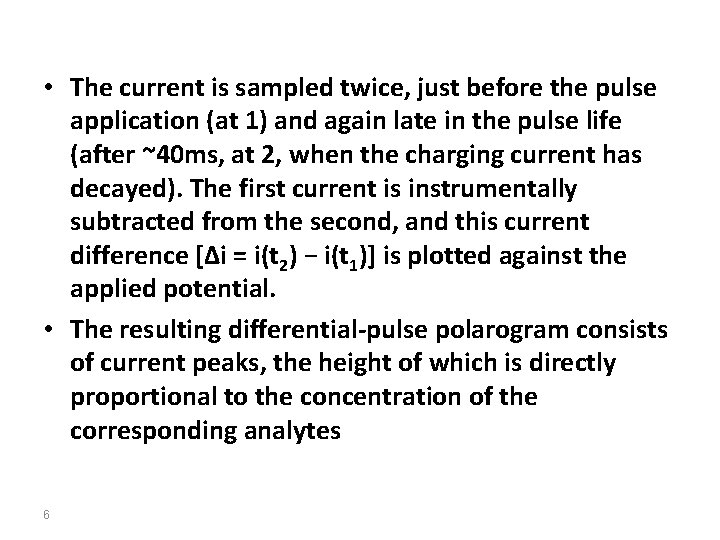
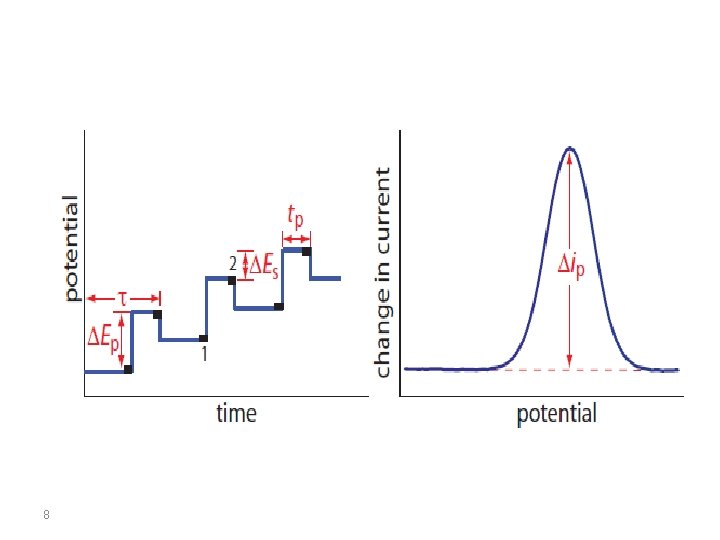
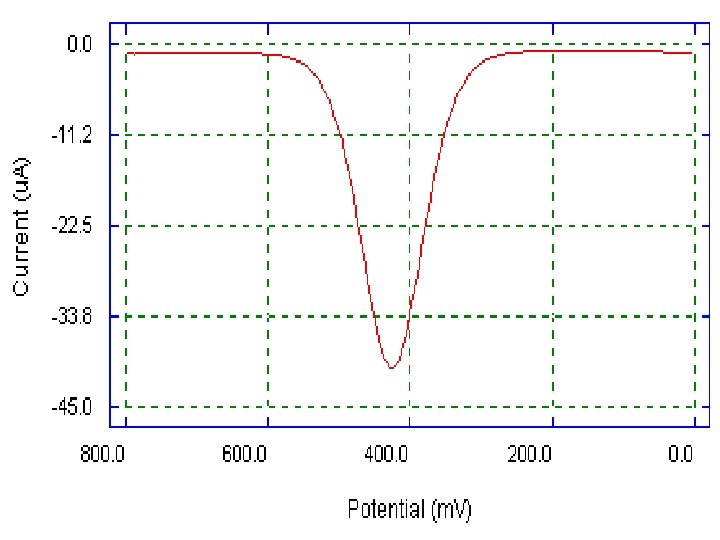
• The current is sampled twice, just before the pulse application (at 1) and again late in the pulse life (after ~40 ms, at 2, when the charging current has decayed). The first current is instrumentally subtracted from the second, and this current difference [Δi = i(t 2) − i(t 1)] is plotted against the applied potential. • The resulting differential-pulse polarogram consists of current peaks, the height of which is directly proportional to the concentration of the corresponding analytes 6

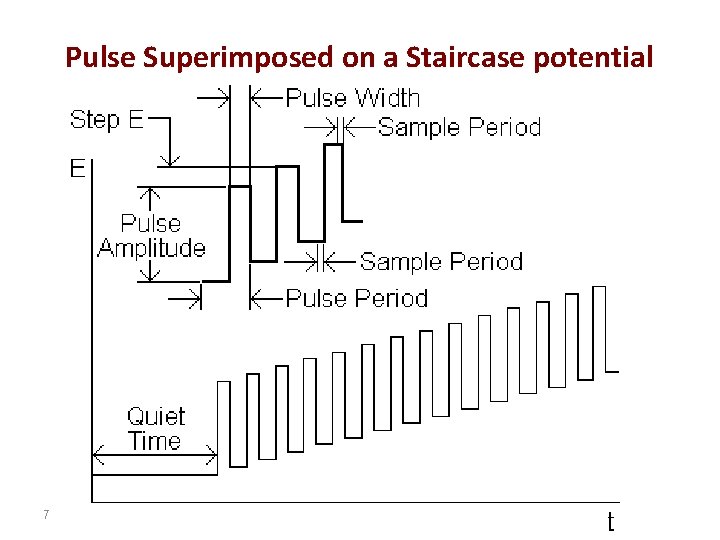
Pulse Superimposed on a Staircase potential 7

8

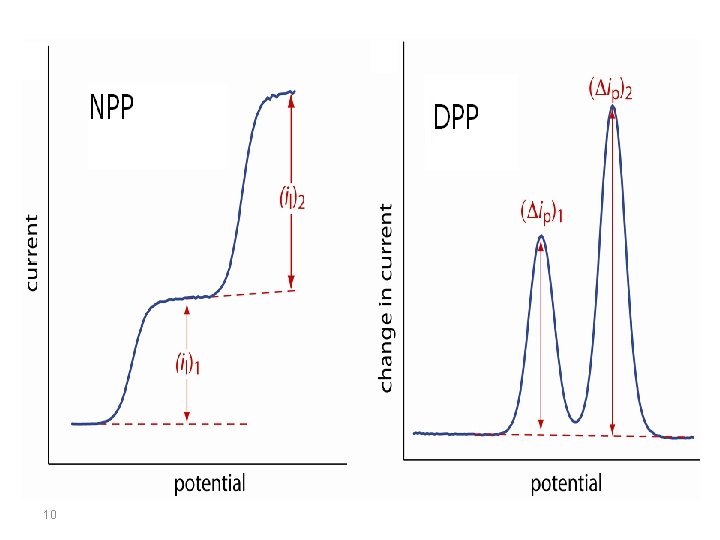
In a reduction, at potentials well positive of the redox potential, there is no faradaic reaction in repsonse to the pulse, so the difference current is zero. At potential around the redox potential, the difference current reaches a maximum, and decreases to zero as the current becomes diffusion-controlled. The current response is therefore a symmetric peak. 9

10

11

12

13

14

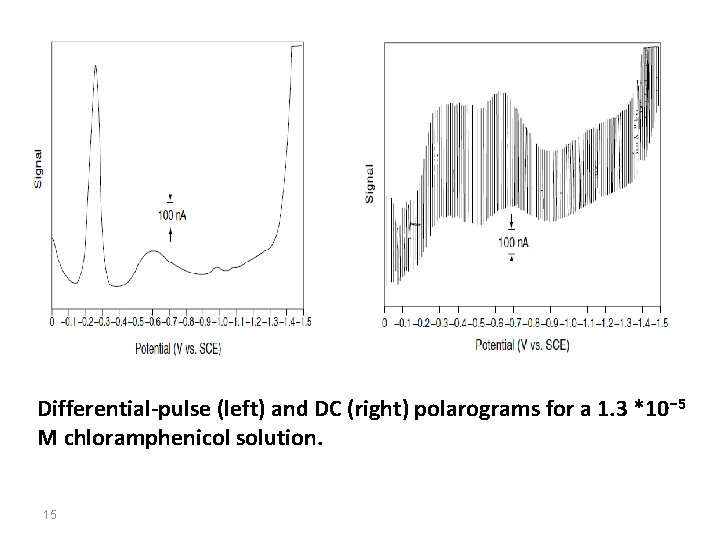
Differential-pulse (left) and DC (right) polarograms for a 1. 3 *10 − 5 M chloramphenicol solution. 15

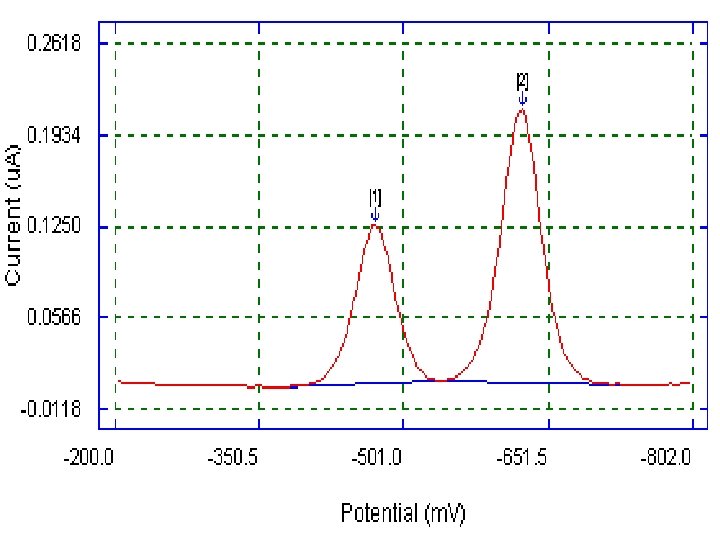
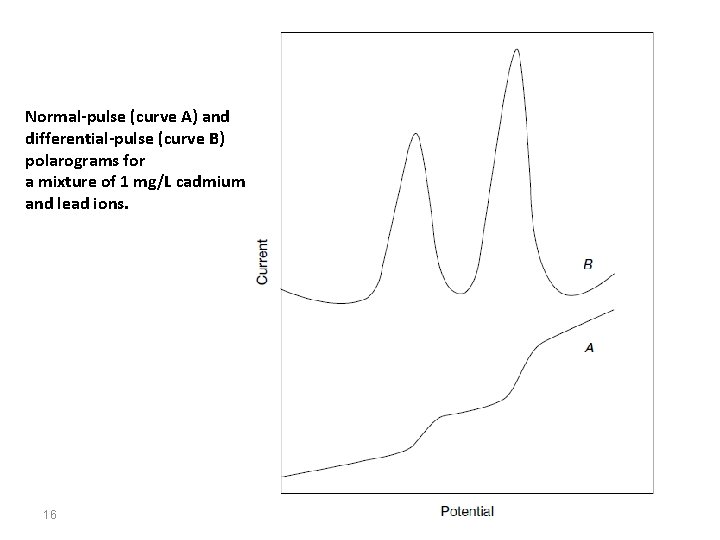
Normal-pulse (curve A) and differential-pulse (curve B) polarograms for a mixture of 1 mg/L cadmium and lead ions. 16

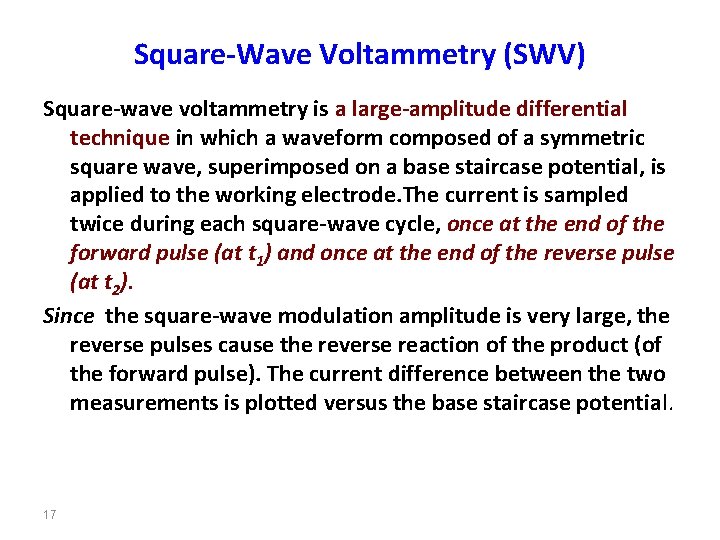
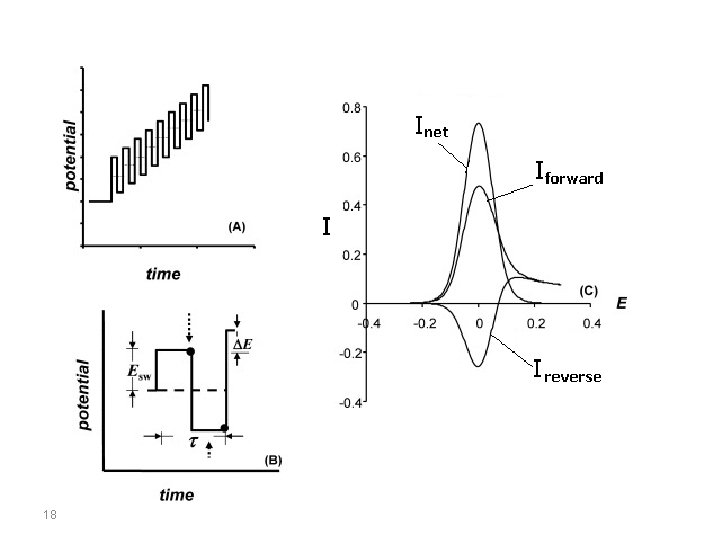
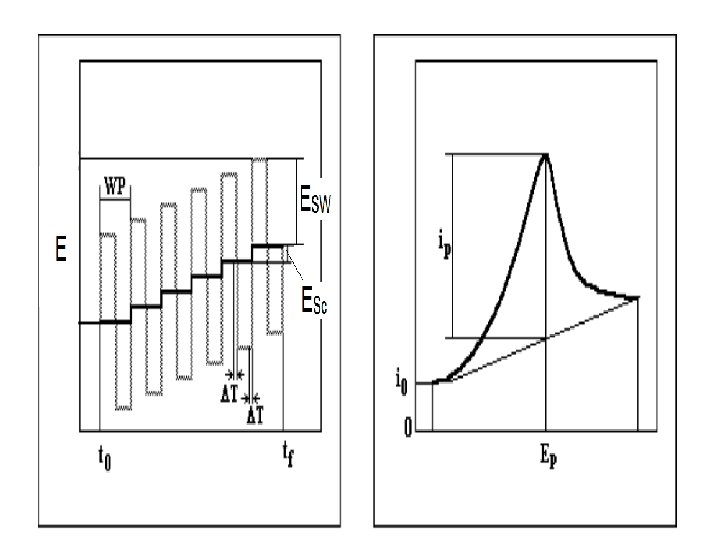
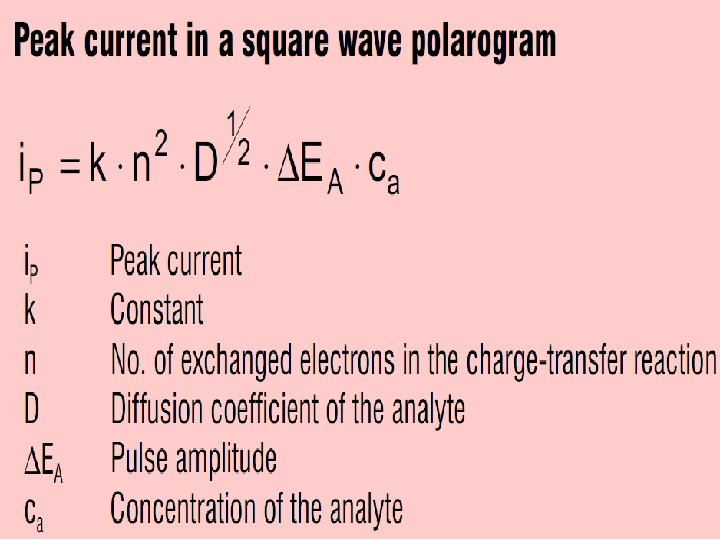
Square-Wave Voltammetry (SWV) Square-wave voltammetry is a large-amplitude differential technique in which a waveform composed of a symmetric square wave, superimposed on a base staircase potential, is applied to the working electrode. The current is sampled twice during each square-wave cycle, once at the end of the forward pulse (at t 1) and once at the end of the reverse pulse (at t 2). Since the square-wave modulation amplitude is very large, the reverse pulses cause the reverse reaction of the product (of the forward pulse). The current difference between the two measurements is plotted versus the base staircase potential. 17

18

19

There are two advantages to measuring the difference current. First, it increases the discrimination against the charging current, since any residual charging current is subtracted out. Second, the shape of the current response is a symmetric peak, rather than the sigmoidal curve typically found for normal pulse voltammetry. 20

If we consider a reduction, then at potential well positive of the redox potential, both the forward and reverse currents are zero, so the difference current is also zero. At potentials well negative of the redox potential, the current is diffusioncontrolled, and the potential pulse has no effect; hence, the forward and reverse currents are equal, and the difference current is again zero. The largest difference between the forward and reverse currents (and hence the largest current response) is at the redox potential. 21

22

23

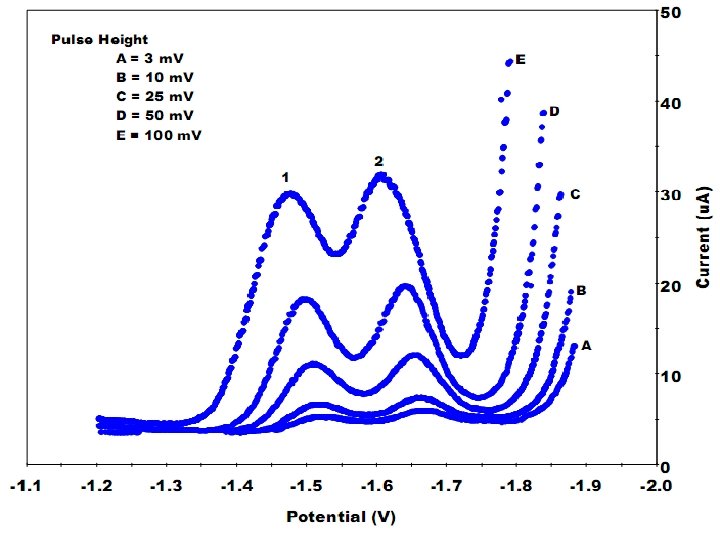
Comments on Performance of SWV 1. The sensitivity of this technique can be increased by enhancing the amplitude of the square wave or the frequency. The limits of the enhancing is strictly related to the kinetics aspects of the redox system. 2. The interference due to capacitive current are lowered to minimum because the current is sampled just at the end of the half waves, when the current of the electrical double layer is least. 3. Detection limits range from 5 – 50 µg/l. 4. The procedure is fast (few seconds) and can be done using one mercury drop (on a DME) or using a HMDE. 24

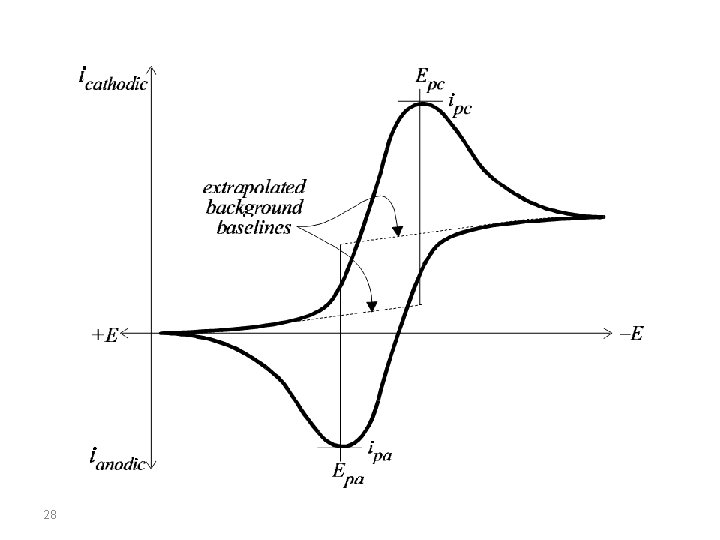
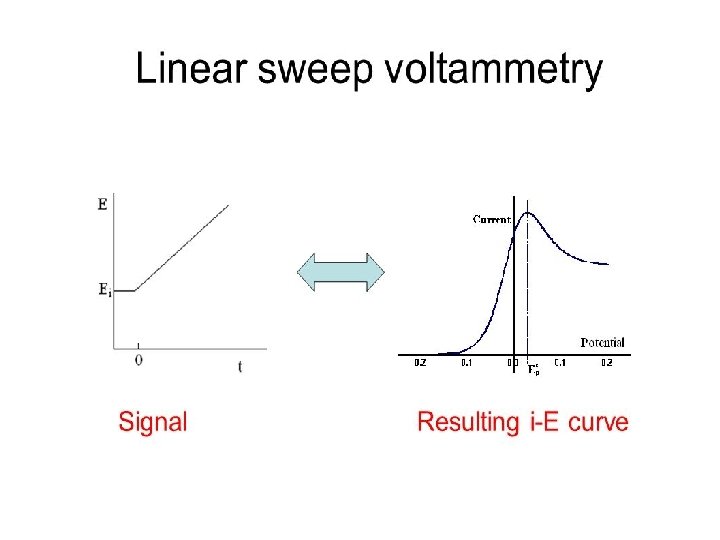
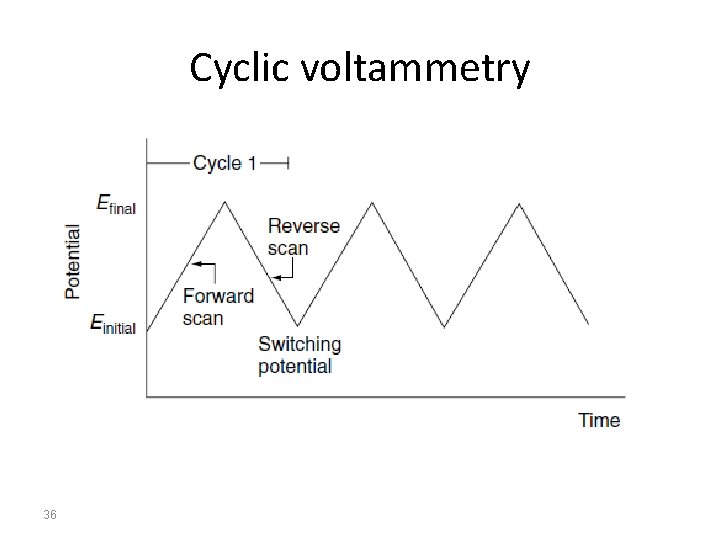
Cyclic Voltammetry Cyclic voltammetry is a very important electrochemical technique it can be used to study the redox behaviour of compounds and to determine mechanisms and rates of oxidation/reduction reactions. Cyclic voltammetry is a simple extension of the linear sweep technique. Conventional cyclic voltammetry is especially informative about the qualitative aspects of an electrode process. The use of a triangular waveform at stationary electrodes, allows both the oxidation and reduction pathways to be studied conveniently from the experiment. 25

Cyclic voltammetry has been established as the electrochemical method of choice for the evaluation of the mechanism of charge transfer for more than five decades. During these years a number of methods have been developed for the measurement of electrode reaction kinetics. Furthermore, the presence of homogeneous reactions in the mechanism is readily detected, and interpretation of results usually is simple. A direct estimate of electrode reversibility is provided, because the potentials at which oxidation and reduction occur are observed directly within range. 26

Conventions The potential is graphed along the x-axis with more positive (or oxidizing) potentials plotted to the left, and more negative (or reducing) potentials to the right. The current is plotted on the y-axis of the voltammogram, with cathodic (i. e. , reducing) currents plotted up along the positive direction, and anodic (i. e. , oxidizing) currents plotted down in the negative direction. (A voltammogram is almost always plotted in this fashion by North American electrochemists, but in Europe, the axes are typically reversed. ) 27

28

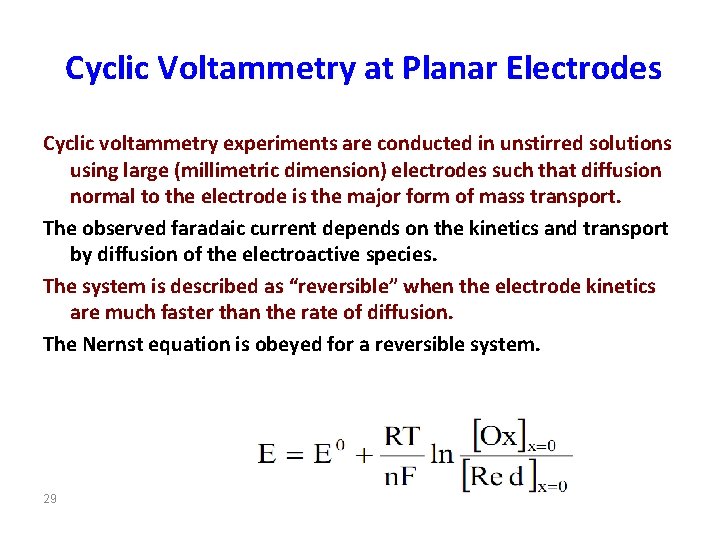
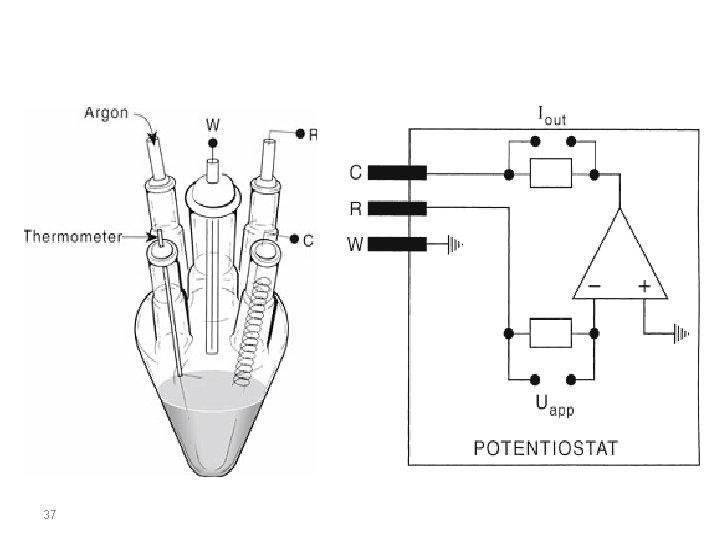
Cyclic Voltammetry at Planar Electrodes Cyclic voltammetry experiments are conducted in unstirred solutions using large (millimetric dimension) electrodes such that diffusion normal to the electrode is the major form of mass transport. The observed faradaic current depends on the kinetics and transport by diffusion of the electroactive species. The system is described as “reversible” when the electrode kinetics are much faster than the rate of diffusion. The Nernst equation is obeyed for a reversible system. 29
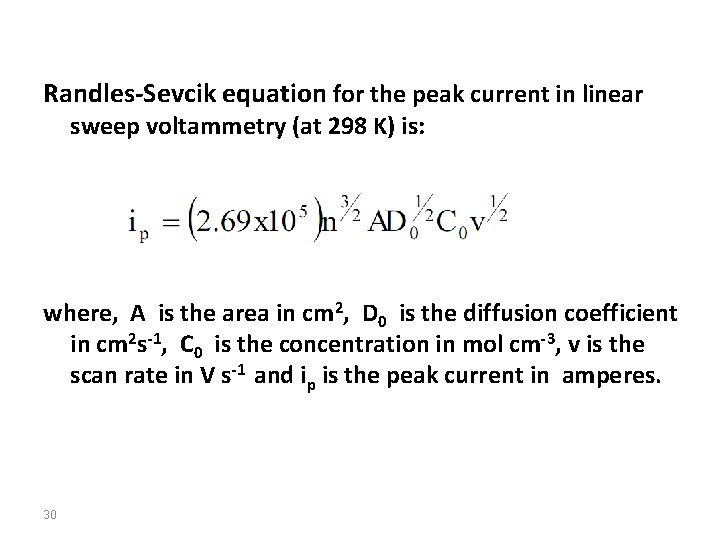
Randles-Sevcik equation for the peak current in linear sweep voltammetry (at 298 K) is: where, A is the area in cm 2, D 0 is the diffusion coefficient in cm 2 s-1, C 0 is the concentration in mol cm-3, v is the scan rate in V s-1 and ip is the peak current in amperes. 30

31

Comparison between Reversible and Irreversible Voltammograms For systems (reversible and irreversible), the peak current i p, increases when the scan rate and the concentration of the reactant species increase. This increase of ip with increasing scan rate is due to fact that the diffusion layer becomes thinner and the concentration gradient steeper. The separation of the peak potentials, Epa - Epc, is an important parameter in cyclic voltammetry which can be used to calculate the reversibility of the reaction. The peak potential separation for a reaction with reversible electron transfer is given by: Epa-Epc = {~59/n} m. V 32

The value of the peak potential separation at 298 K is approximately 56. 5 m. V and is independent of scan rate. A ratio of the peak currents, ipa/ipc which is one for reversible systems, is another important parameter in these systems. It is independent of the scan rate. On the other hand in irreversible systems, the ratio of the peak currents, ipa/ipc and the reverse peak potential are dependent on scan rate. In order to force the reverse electron transfer to occur in the short time scale used, a high overpotential is essential; therefore the peak separation (E pa -Epc) is dependent on the irreversibility of the system. 33

Judging Reversibility from CV 1. 2. 3. 4. 5. 6. n DEpeak ~ 59 m. V ipa / ipc = 1 Eo = (Epa + Epc) / 2 ipa and ipc a (scan rate)1/2 Epeak independent on scan rate Half wave potential: Sign +ve for reduction and –ve for oxidation. Quantitative information regarding analyte concentration can be obtained from the voltammogram using the Randles-Sevcik equation seen before (v is the sweep rate): 34

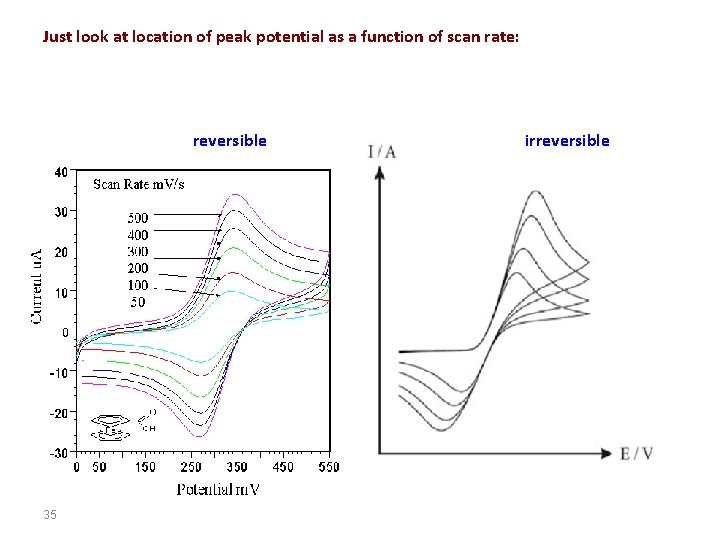
Just look at location of peak potential as a function of scan rate: reversible 35 irreversible

Cyclic voltammetry 36

37