Volcanic eruptions Eruptions put large amounts of sulfur















- Slides: 29

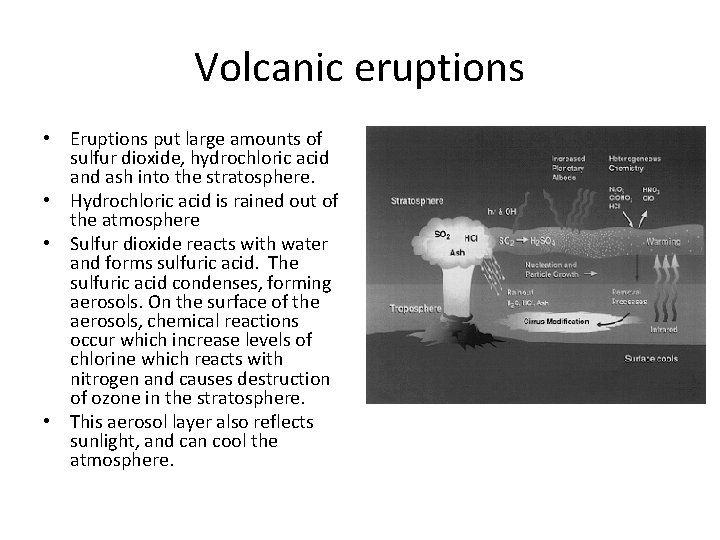
Volcanic eruptions • Eruptions put large amounts of sulfur dioxide, hydrochloric acid and ash into the stratosphere. • Hydrochloric acid is rained out of the atmosphere • Sulfur dioxide reacts with water and forms sulfuric acid. The sulfuric acid condenses, forming aerosols. On the surface of the aerosols, chemical reactions occur which increase levels of chlorine which reacts with nitrogen and causes destruction of ozone in the stratosphere. • This aerosol layer also reflects sunlight, and can cool the atmosphere.

Fly Ash • Residue of coal burning • Contains silicon dioxide, calcium oxide, arsenic, beryllium, boron, cadmium, chromium VI, cobalt, lead, manganese, mercury, molybdenum, selenium, strontium, thallium, and vanadium, along with dioxins and PAH compounds

Fly Ash • In the past, was released into the atmosphere. • In the US, it is now it is captured and stored at the coal plants or in landfills • 43% is recycled and used in cement, grout, roofing tiles, paints, metal casings, filler in wood and plastic products. • Contains radioactive elements including uranium, thorium and potassium as well as their decay products

Storage problems • Kingston TN, Dec 23, 2008. Containment failure at the TVA plant. • Not classified as a hazardous waste by the EPA • Some concern that the level of radioactivity in the fly ash is equal to or more than you receive living near a BWR nuclear facility-still below safety limits

Effects of particulate matter • Size is critical • Your body has mechanisms designed to filter out particles with sizes larger than 1 um. (nose, mucous membranes, sneezing and coughing. • However, smaller particles can pass into the lungs and cause problems

Effects of particulate matter Respiratory problems Lung cancers Particles can carry other toxic substance Health begins to deteriorate for long term exposure at levels of 80 ug/m 3. • Decrease in sunlight-cities with high levels of population and industry receive 20% less sunlight than areas with less population and industry. • Buildings and clothing damaged • Toxic substances in the particulates can contribute to decay of masonry and corrosion of metals • •

Removal of particulates • Natural processes –particles stay in the atmosphere days to years – Gravity – Precipitation • small particles form nuclei around which raindrops form • Rain washes out particulates • Natural processes cannot keep up with the rate of man made particulate pollution

Removal of particulates • Removal of ash from coal burning – 2 types of ash • Bottom ash-heavy, sinks to the bottom of the boilers • Fly ash –light goes up the stack and released in to the atmosphere • Bag house- air passes through a set of vibrating filters. The vibrations shake the particles out of the filter and they sink to the bottom for collection

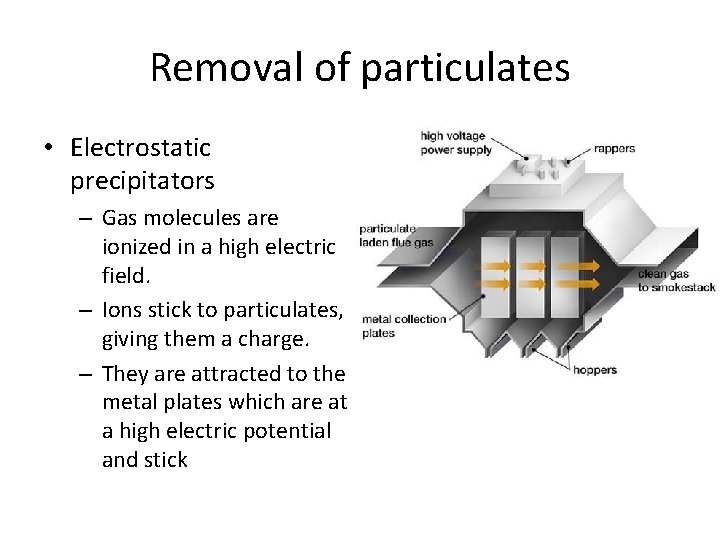
Removal of particulates • Electrostatic precipitators – Gas molecules are ionized in a high electric field. – Ions stick to particulates, giving them a charge. – They are attracted to the metal plates which are at a high electric potential and stick

Removal of particulates • Cyclone separators – Gas is circulated in a circular motion – Particles hit the walls and settle out – Not very efficient for small particles

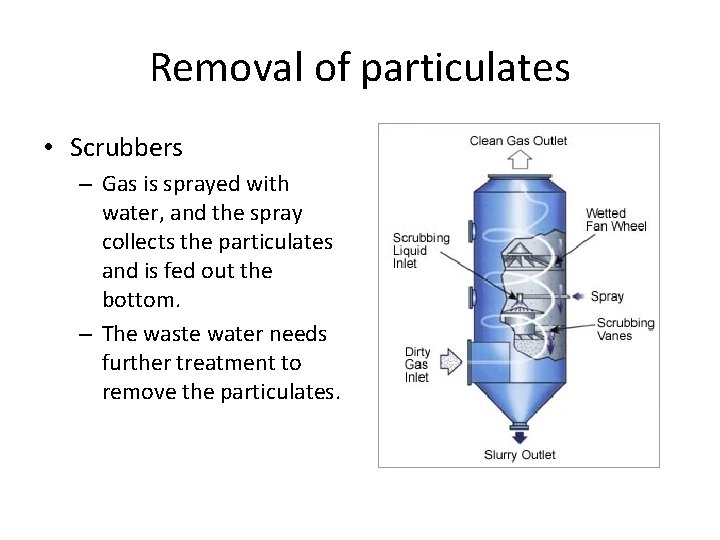
Removal of particulates • Scrubbers – Gas is sprayed with water, and the spray collects the particulates and is fed out the bottom. – The waste water needs further treatment to remove the particulates.

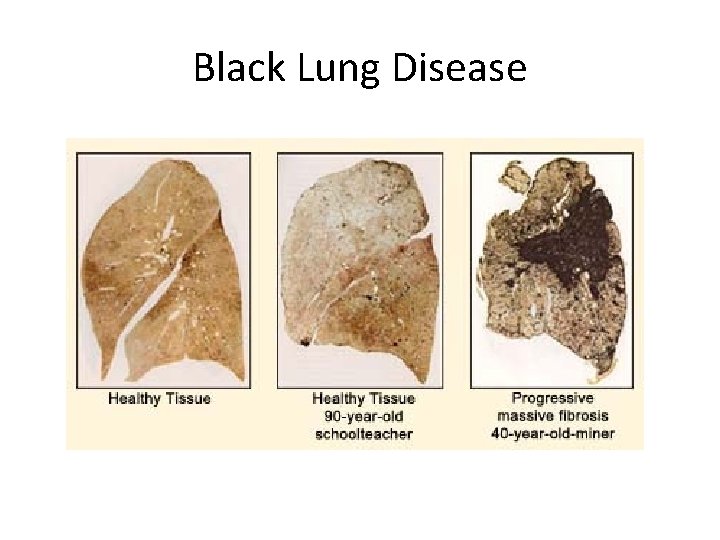
Black Lung Disease • Also called coal workers' pneumoconiosis • Coal dust particles are too small to be filtered by the bodies natural filtration systems • Once in the body, it cannot be removed or destroyed – In the lungs, it is engulfed by white blood cells known as macrophages and it stays in the lungs or lymph nodes • Aggregations of these carbon filled macrophages form which cause inflammation, fibrosis and lesions • Lung efficiency is reduced, leading to other complications such as respiratory or heart failure • http: //www. courier-journal. com/cjextra/blacklung/index. html -A Louisville Courier Journal article on the state of Black Lung Disease in KY • Responsible for 1500 deaths in the US each year

Black Lung Disease

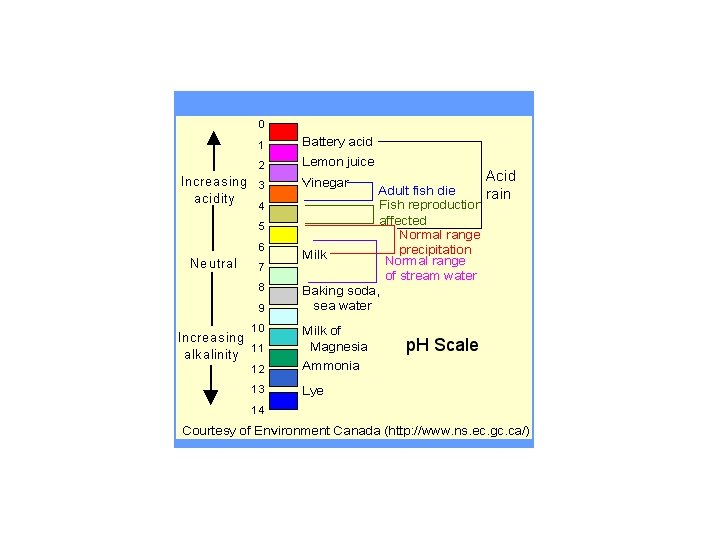
Acid Rain • How do we measure acidity of a liquid (water in this case? ) • p. H-measure of acidity, ranges from 0 to 14. • p. H =7. 0 is neutral • p. H < 7. 0 is acidic-there is an excess of Hydrogen ions • p. H > 7. 0 is alkaline –there is a lack of H ions • Scale is logarithmic-this means that a change in 1 on the scale equals a change of a factor of 10 in the H ion concentration.


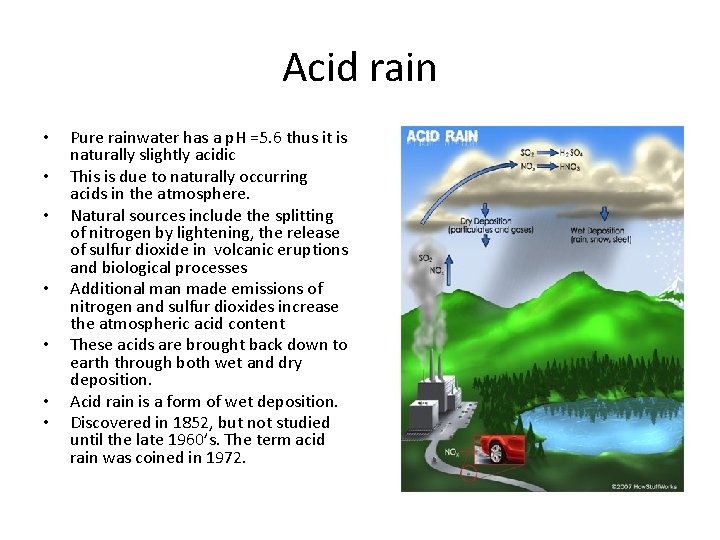
Acid rain • • Pure rainwater has a p. H =5. 6 thus it is naturally slightly acidic This is due to naturally occurring acids in the atmosphere. Natural sources include the splitting of nitrogen by lightening, the release of sulfur dioxide in volcanic eruptions and biological processes Additional man made emissions of nitrogen and sulfur dioxides increase the atmospheric acid content These acids are brought back down to earth through both wet and dry deposition. Acid rain is a form of wet deposition. Discovered in 1852, but not studied until the late 1960’s. The term acid rain was coined in 1972.

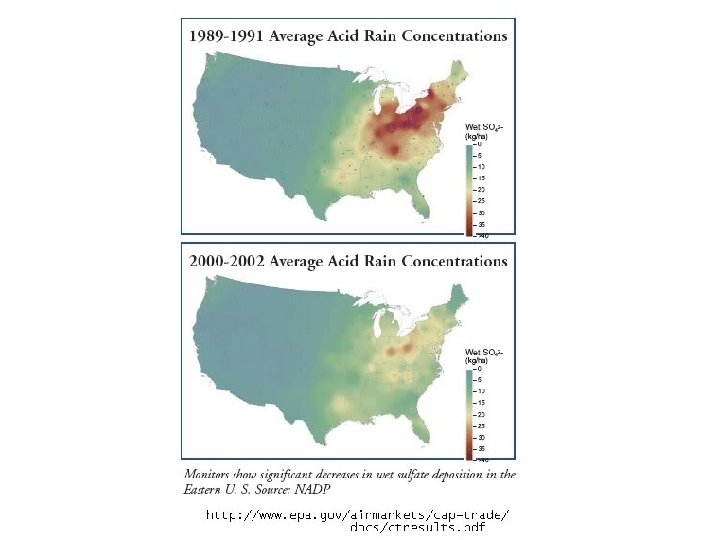
Acid rain • Main constituent is sulfur dioxide • Main producer in North America are the coal burning plants in the midwest • Effect is seen mostly in the lakes of upstate New York, northern New England, Ontario and Quebec. • US is the largest producer of sulfur dioxide in the world.



Effects of Acid rain • Depends upon wind patterns (plants in the Midwest US effect upstate New York, northern New England, etc). • Depends upon local bedrock • Limestone neutralizes the acid • Granite and other common bedrocks do little to neutralize the acid. • Water looks normal, but p. H is lowered. • Newly hatched fish cannot survive, other fish suffocate due to aluminum that is released from the soil because of the lower p. H, which clogs their gills. • Other reptiles, plants and insects suffer the same fate • Eventually, the only life the lake can support is algae growing on its bottom. • The disruption to this portion of the ecosystem trickles down to other organisms that depend upon the life in the lake to support them.

Effect of acid snow • Can be worse than acid rain • Can result in an acid shock • As snow melts and runs off into the rivers and streams • p. H can be decreased by 2 units in a couple of weeks-amounts to hundred fold increase in the H ion concentration (remember the p. H scale is logarithmic)

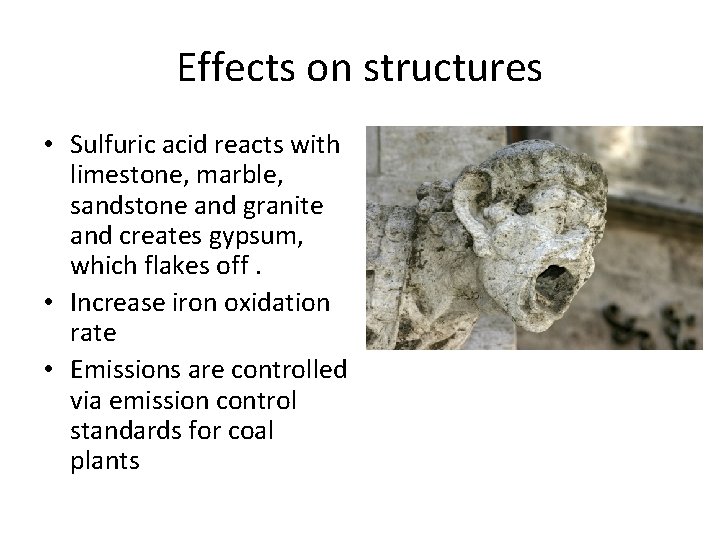
Effects on structures • Sulfuric acid reacts with limestone, marble, sandstone and granite and creates gypsum, which flakes off. • Increase iron oxidation rate • Emissions are controlled via emission control standards for coal plants

Solutions • Techniques to remove sulfur either from the flue as we discussed last lecture or by detecting and diverting high sulfur content coal before it is burned. • Automobile emission control systems reduce emissions of nitrogen oxides

Solutions • Clean Air Act amendments of 1990 established the Acid Rain Program • Wide support in Congress and signed by president George H W Bush. • ARP implemented what is known as emissions trading (Cap and Trade) • http: //www. smithsonianmag. com/science-nature/Presence -of-Mind-Blue-Sky-Thinking. html? c=y&page=1 • Emission caps are set, and producers are given an emission allowance. If they institute controls and come in under that allowance, they can sell of their allowance to another producer who is not meeting their targets.

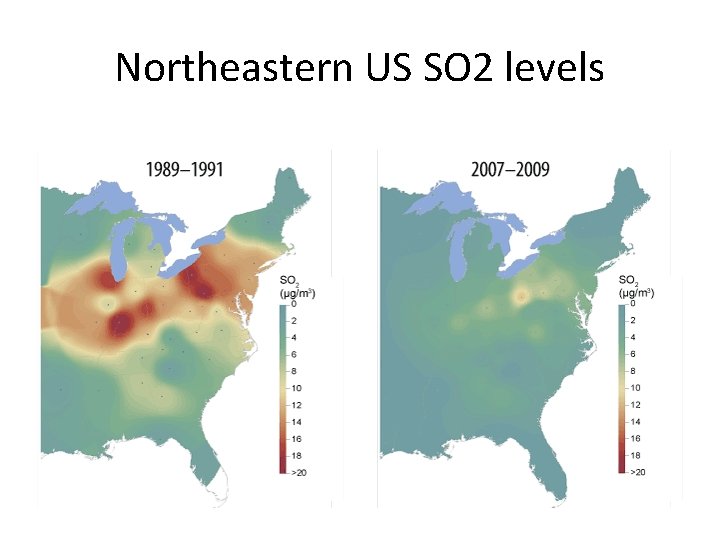
Effectiveness • Considered a success by EPA, industry, economists and certain environmental groups. • Skeptics argue that reduction in emissions occurred due to broad trends unconnected to the program • Since the 1990 s, SO 2 emissions have dropped 40%, and acid rain levels have dropped 65% since 1976. • 2007, total SO 2 emissions were 8. 9 million tons, achieving the program's long term goal ahead of the 2010 statutory deadline • In 2008, SO 2 dropped to 7. 6 million tons

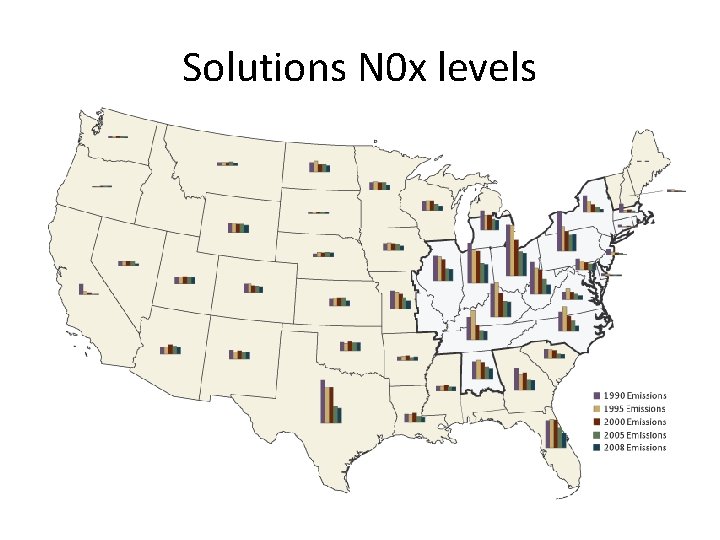
Solutions N 0 x levels

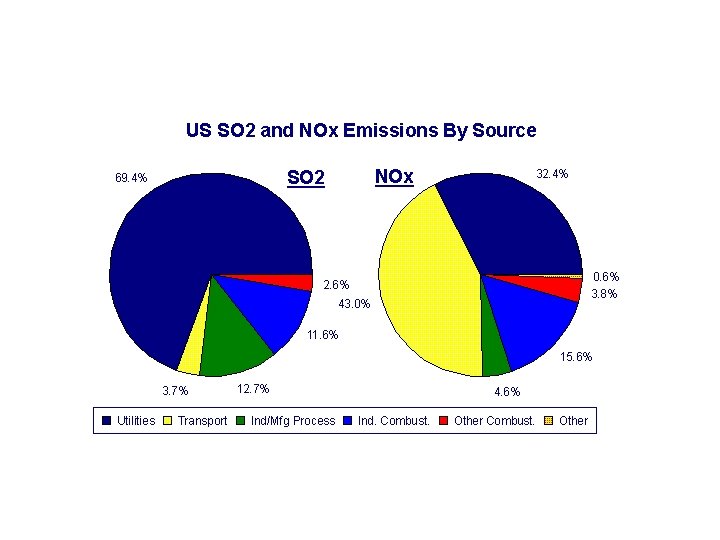
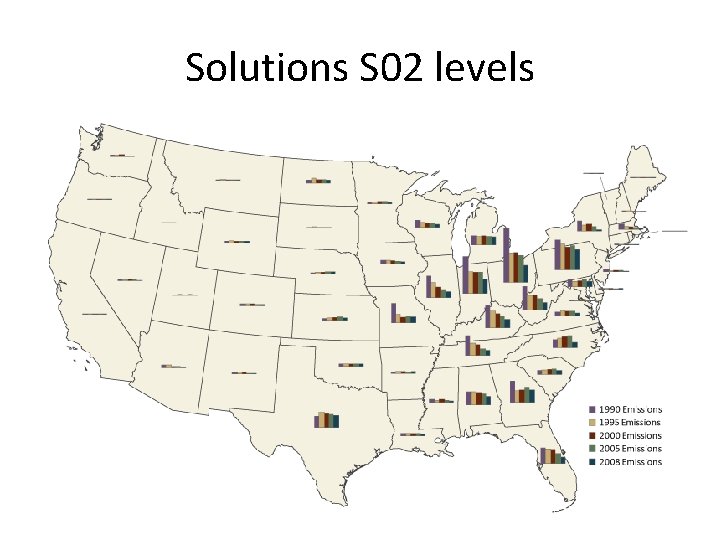
Solutions S 02 levels


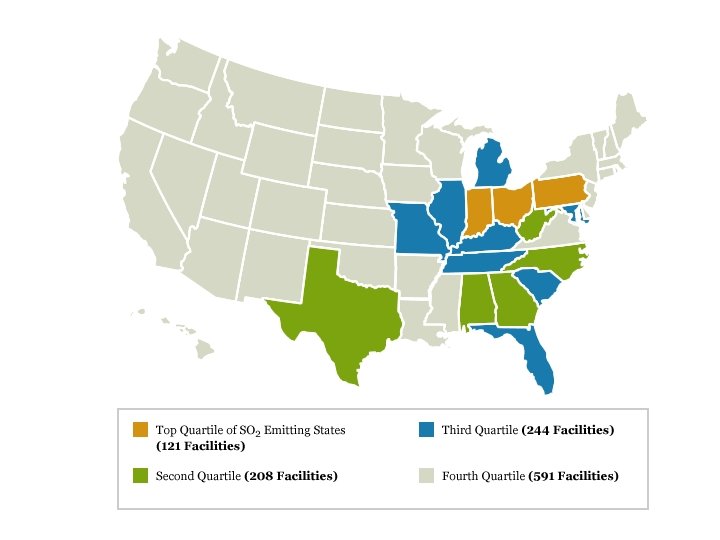
Northeastern US SO 2 levels