Vocabulary of PT Columns called groups or families


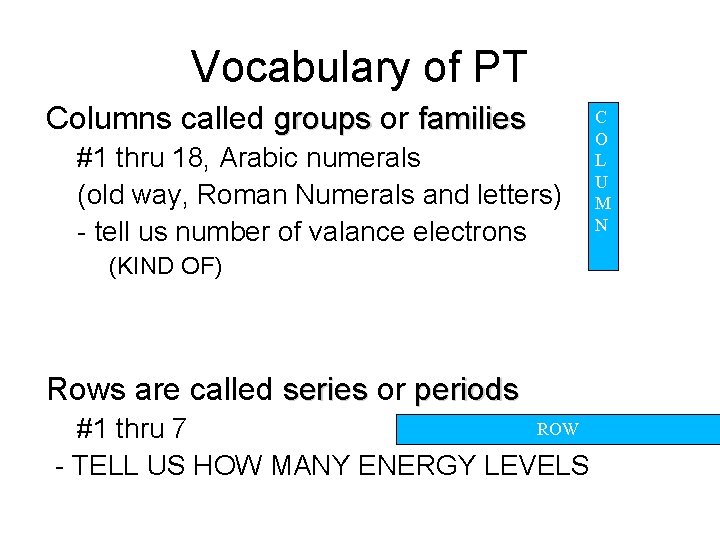
Vocabulary of PT • Columns called groups or families – #1 thru 18, Arabic numerals – (old way, Roman Numerals and letters) – - tell us number of valance electrons • (KIND OF) • Rows are called series or periods ROW – #1 thru 7 - TELL US HOW MANY ENERGY LEVELS C O L U M N

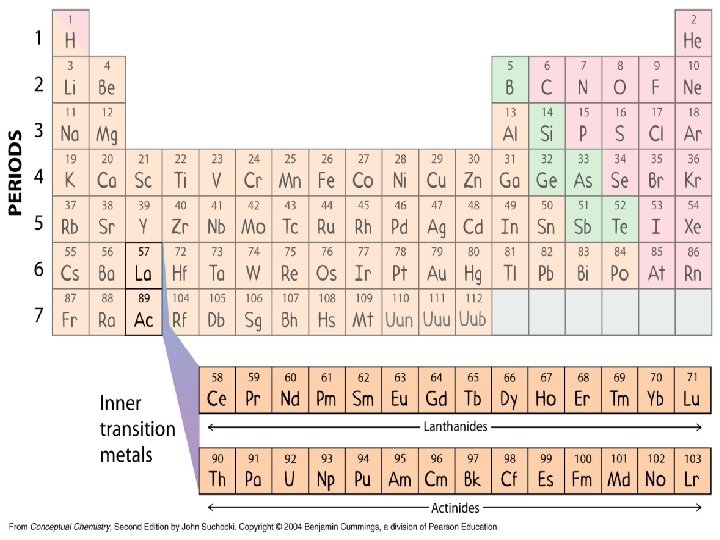
ROWS Period numbering (1 7) top to bottom

Energy Levels = Row Number • Closely related to electron configuration of each element • Elements in same row have same # of principal energy levels – so # of principal energy levels = to row #
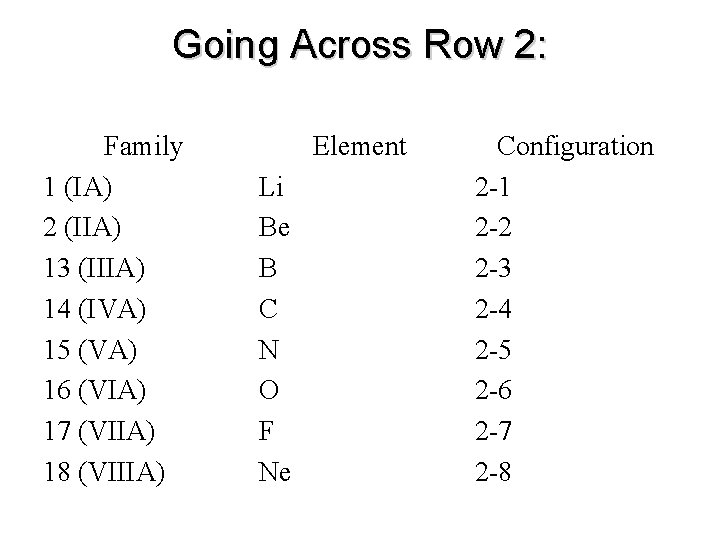
Going Across Row 2: Family 1 (IA) 2 (IIA) 13 (IIIA) 14 (IVA) 15 (VA) 16 (VIA) 17 (VIIA) 18 (VIIIA) Element Li Be B C N O F Ne Configuration 2 -1 2 -2 2 -3 2 -4 2 -5 2 -6 2 -7 2 -8
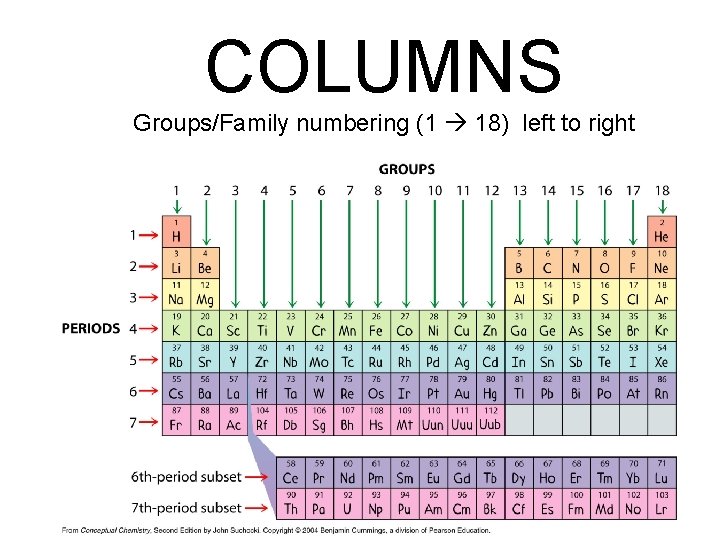
COLUMNS Groups/Family numbering (1 18) left to right
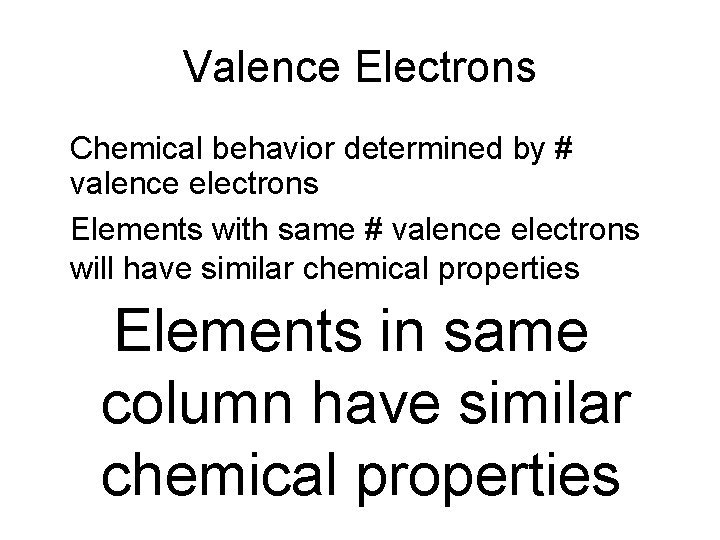
Valence Electrons • Chemical behavior determined by # valence electrons • Elements with same # valence electrons will have similar chemical properties –Elements in same column have similar chemical properties
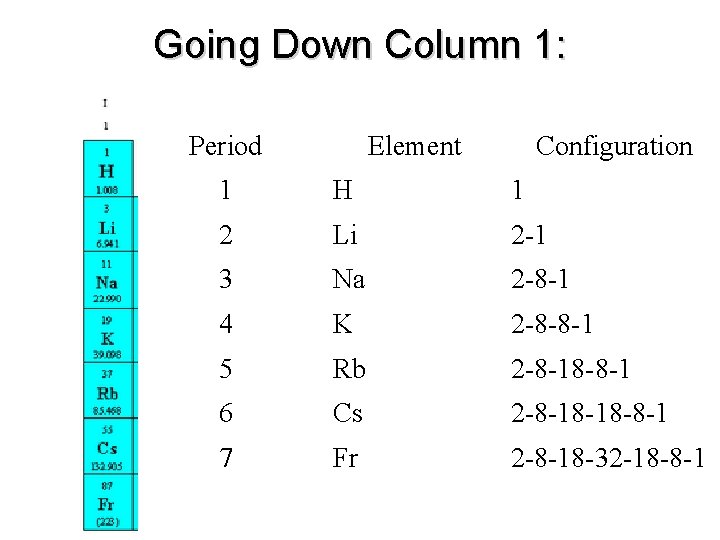
Going Down Column 1: Period Element Configuration 1 H 1 2 Li 2 -1 3 Na 2 -8 -1 4 K 2 -8 -8 -1 5 Rb 2 -8 -18 -8 -1 6 Cs 2 -8 -18 -18 -8 -1 7 Fr 2 -8 -18 -32 -18 -8 -1
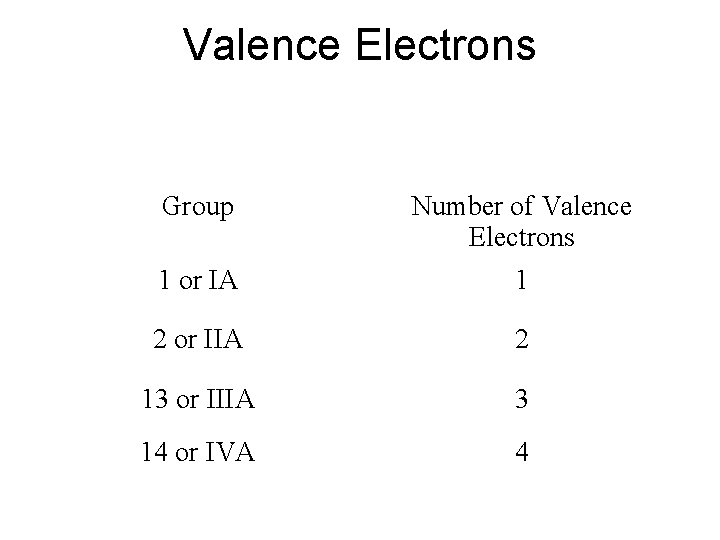
Valence Electrons Group Number of Valence Electrons 1 or IA 1 2 or IIA 2 13 or IIIA 3 14 or IVA 4

Names of Families (AKA group A elements) • • • Group 1 = Alkali Metals Group 2 = Alkaline Earth Metals Groups 3 -12: Transition metals Group 13 = Boron family Group 14 = Carbon family Group 15 = Nitrogen family Group 16 = Oxygen family Group 17 = Halogens Group 18 = Noble Gases
- Slides: 13