Vocabulary Ch 2 Sec 2 Units of Measurement
Vocabulary- Ch. 2, Sec. 2 Units of Measurement • Measurement: dimensions, quantity, or capacity as ascertained by ________; they represent _______. • Quantity: something that has ____, or ______ • Measurement and quantity are _____ the same. • Example: the quantity represented by a liter is ______, the liter itself is ___________.
Standard Measurement • Nearly every measurement is a number plus a unit – Note: Is is going to be very helpful if you train yourself to keep up with numbers and units in calculations. • There has to be an agreement on measurement standards so comparisons can be made.

“SI” Measurement • Le Systeme International d’Unites – French • Adopted in 1960 • Has seven base units with other units ______ from these seven • Are standard measurements • Often called ______but there actually some differences.
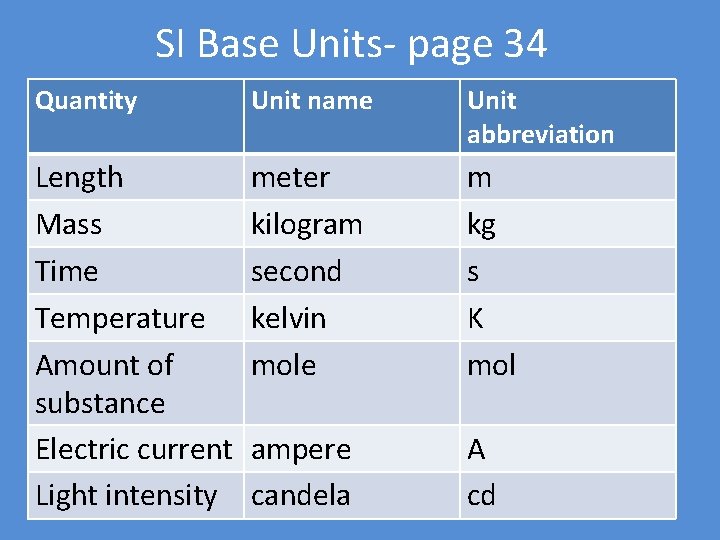
SI Base Units- page 34 Quantity Unit name Unit abbreviation Length Mass Time Temperature Amount of substance Electric current Light intensity meter kilogram second kelvin mole m kg s K mol ampere candela A cd
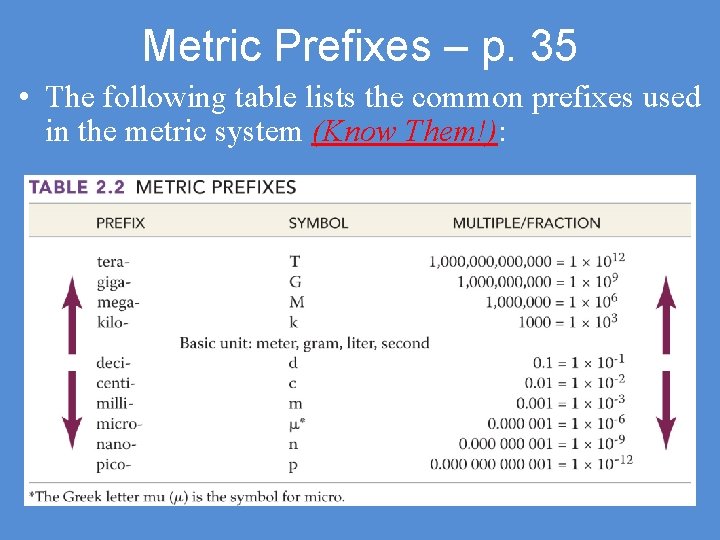
Metric Prefixes – p. 35 • The following table lists the common prefixes used in the metric system (Know Them!):


Derived SI Units (page 36) • Volume: _____________ – V = l x w x h (gives us cubic distance) • The derived SI unit of volume is __ (___ is often used in chemistry) • A non-SI unit of volume is the ______, L (smaller non-SI unit of volume is the m. L) • _______
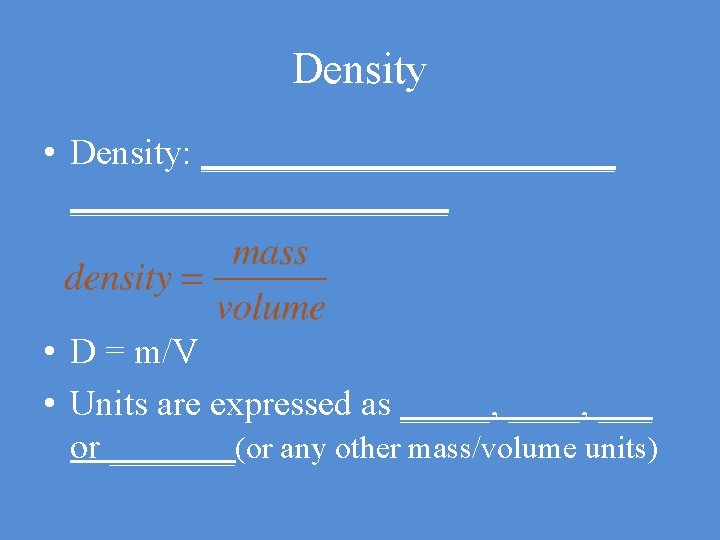
Density • Density: ____________ • D = m/V • Units are expressed as _____, ___ or _______(or any other mass/volume units)

More about Density • Density is a characteristic _______ property • It is an _______property • Can be used to help identify a substance • See the table on page 38 • Density Problems Worked in Class

Conversion Factors • A conversion factor is a _______ derived from the _______ between two different units that can be used to _______ from one unit to the other – They are equalities in _______form. • The following steps will help you use conversion factors for the rest of chemistry…. they don’t ever go away!

Conversions • An effective method for solving problems in science is the _________. • It is also often called _________or the __________. • There are three steps to solving problems using the unit analysis method. – Read the problem and determine the unit required in the answer. – Analyze the problem and determine the given value that is related to the answer. – Write one or more unit factors to convert the unit in the given value to the unit in the answer.

More Practice Convert: 1. 843 m to km 96. 3 hg to g 0. 0013 Mg to mg (two step problem, go to grams first, then to mg) • 4863 µL to L • 1. 4 x 105 g to µg • •
- Slides: 12