Vitrification of Embryos BASAK BALABAN American Hospital of

Vitrification of Embryos BASAK BALABAN American Hospital of Istanbul Assisted Reproduction Unit AMERICAN HOSPITAL TJOD Meeting, Antalya Turkey 2013

Cryopreservation Techniques • Slow conventional freezing • Traditional Vitrification • Ultrarapid vitrification

What is vitrification? • Vitrification is the reversible transition of a liquid into an amorphous noncrystalline glass • Slow freezing uses low concentrations of CPAs (~ 10%), cooling rates of ≤ 1˚C/min, and warming rates of ~250 ˚C/min • Vitrification uses high con. of CPAs (30 -40%), use of saccharides as supplements, cooling rates >1000 ˚C/min(usually >10. 000 ˚C/min) , and very rapid warming rates
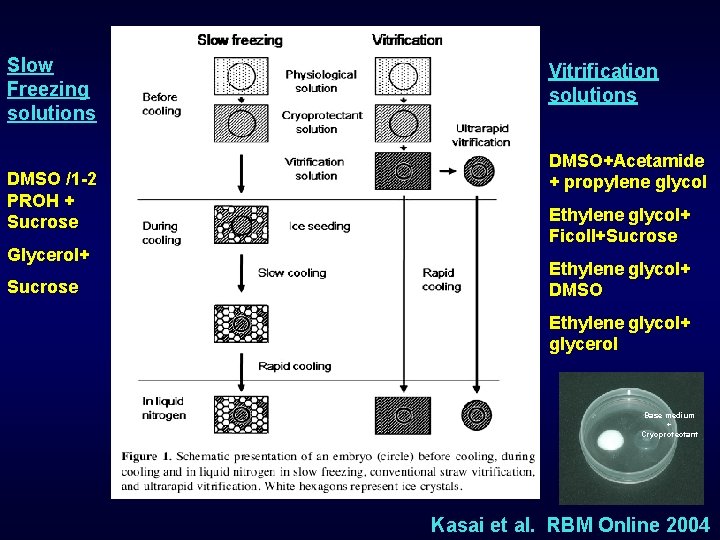
Slow Freezing solutions DMSO /1 -2 PROH + Sucrose Glycerol+ Sucrose Vitrification solutions DMSO+Acetamide + propylene glycol Ethylene glycol+ Ficoll+Sucrose Ethylene glycol+ DMSO Ethylene glycol+ glycerol Base medium + Cryoprotectant Kasai et al. RBM Online 2004
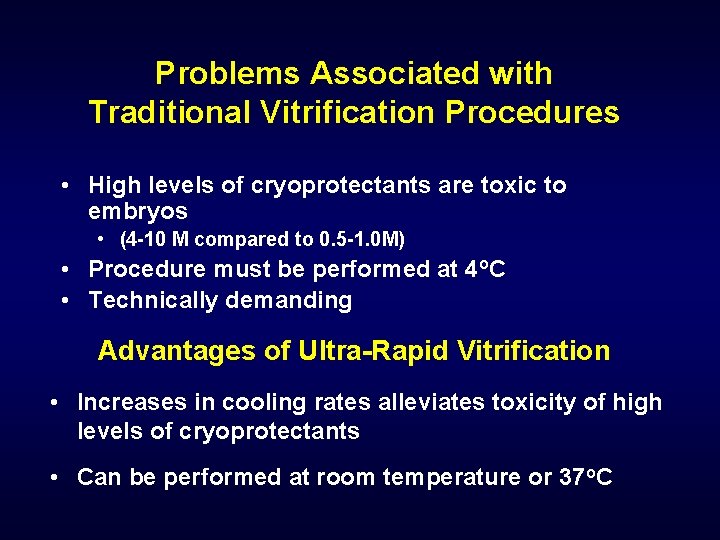
Problems Associated with Traditional Vitrification Procedures • High levels of cryoprotectants are toxic to embryos • (4 -10 M compared to 0. 5 -1. 0 M) • Procedure must be performed at 4 o. C • Technically demanding Advantages of Ultra-Rapid Vitrification • Increases in cooling rates alleviates toxicity of high levels of cryoprotectants • Can be performed at room temperature or 37 o. C
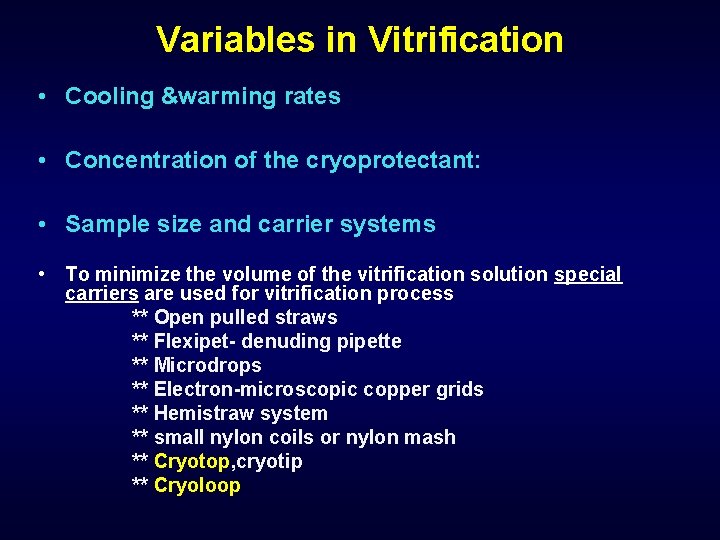
Variables in Vitrification • Cooling &warming rates • Concentration of the cryoprotectant: • Sample size and carrier systems • To minimize the volume of the vitrification solution special carriers are used for vitrification process ** Open pulled straws ** Flexipet- denuding pipette ** Microdrops ** Electron-microscopic copper grids ** Hemistraw system ** small nylon coils or nylon mash ** Cryotop, cryotip ** Cryoloop

Concerns with Regards to Sterility of Liquid N 2 storage • No publication since 1985 had shown cross contamination with viruses, after screening over 450 publications Tedder et al. , 1995 Hepatitis B transmission Bielanski et al. , 2000 Viral contamination Bielanski et al. , 2003 Microbial contamination, no viral cross contamination Kyuma et al. , 2003 No microbial or viral cross contamination
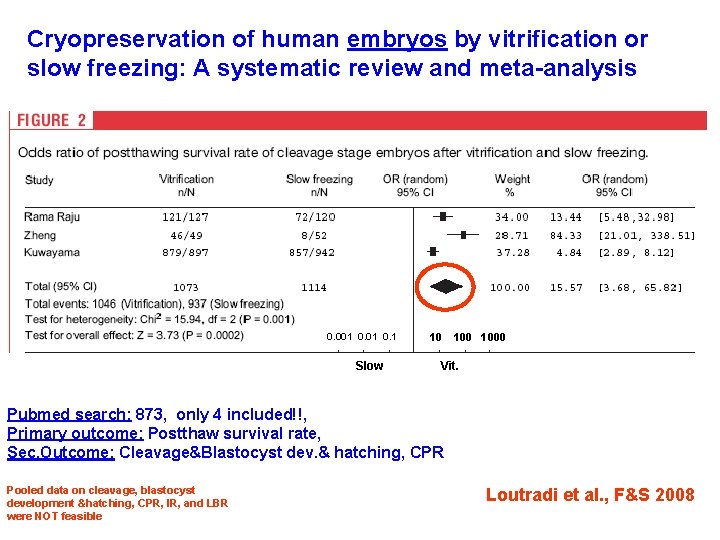
Cryopreservation of human embryos by vitrification or slow freezing: A systematic review and meta-analysis 0. 001 0. 1 Slow 10 1000 Vit. Pubmed search: 873, only 4 included!!, Primary outcome: Postthaw survival rate, Sec. Outcome: Cleavage&Blastocyst dev. & hatching, CPR Pooled data on cleavage, blastocyst development &hatching, CPR, IR, and LBR were NOT feasible Loutradi et al. , F&S 2008
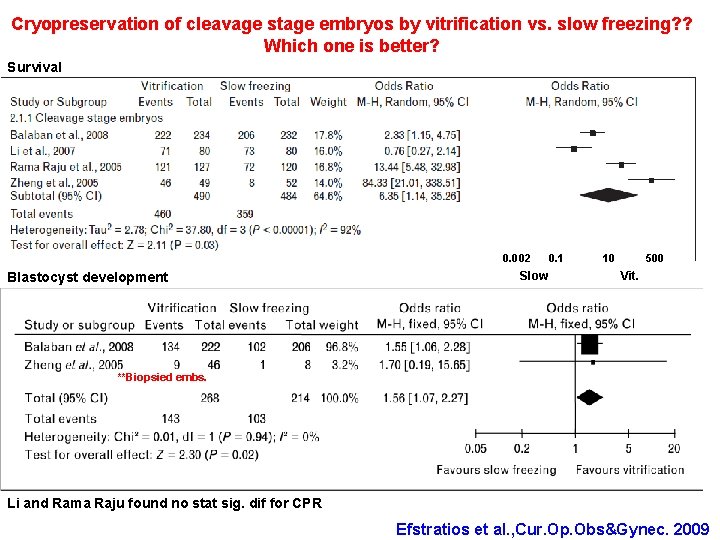
Cryopreservation of cleavage stage embryos by vitrification vs. slow freezing? ? Which one is better? Survival 0. 002 Blastocyst development Slow 0. 1 10 500 Vit. **Biopsied embs. Li and Rama Raju found no stat sig. dif for CPR Efstratios et al. , Cur. Op. Obs&Gynec. 2009
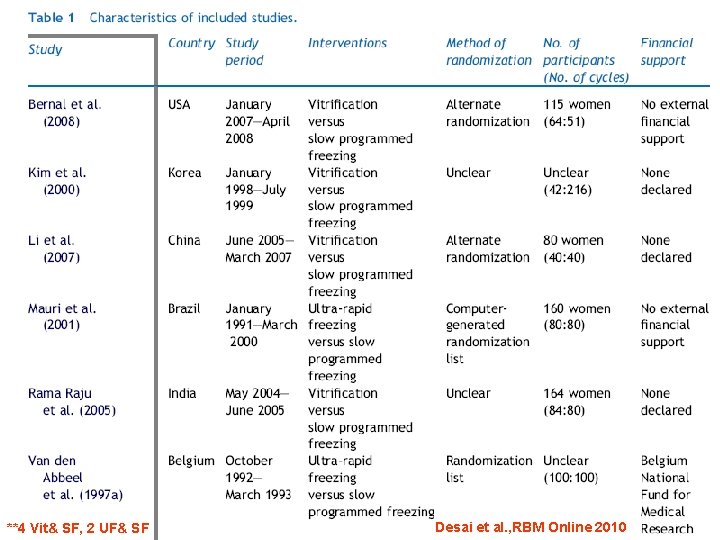
**4 Vit& SF, 2 UF& SF Desai et al. , RBM Online 2010
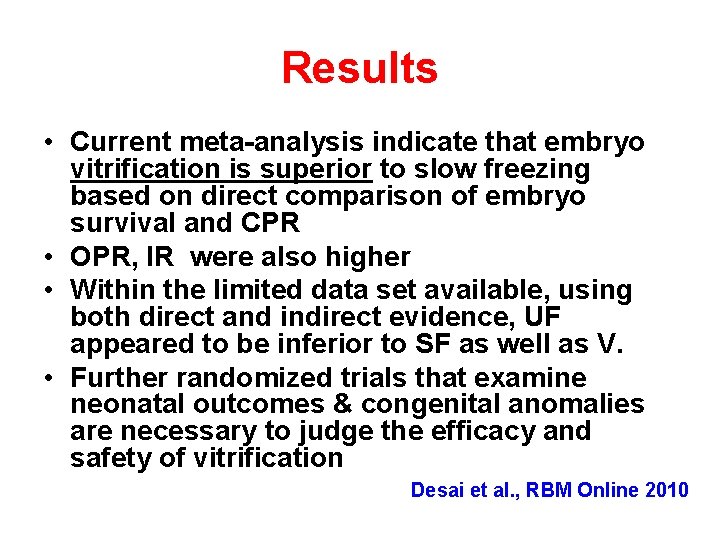
Results • Current meta-analysis indicate that embryo vitrification is superior to slow freezing based on direct comparison of embryo survival and CPR • OPR, IR were also higher • Within the limited data set available, using both direct and indirect evidence, UF appeared to be inferior to SF as well as V. • Further randomized trials that examine neonatal outcomes & congenital anomalies are necessary to judge the efficacy and safety of vitrification Desai et al. , RBM Online 2010
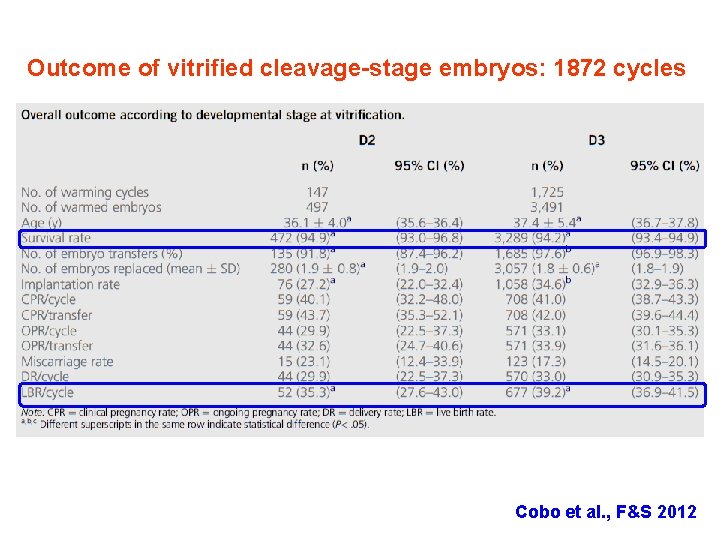
Outcome of vitrified cleavage-stage embryos: 1872 cycles Cobo et al. , F&S 2012

A randomized controlled study on human day 3 embryo cryopreservation by slow freezing or vitrification: vitrification is associated with higher survival, metabolism and blastocyst formation • To compare the blastocyst development between embryos that were cryopreserved by either slow freezing or vitrification on day 3 • To determine whether slow freezing and vitrification have different effects on human cleavage stage embryo metabolism Human Reproduction 2008 Balaban B¹, Urman B¹, Ata B, Isiklar A¹, Larman MG², Hamilton B² and Gardner DK²
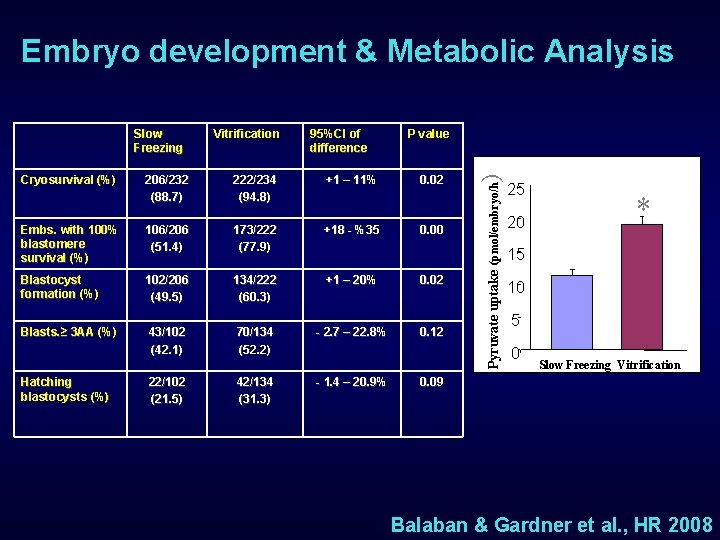
Embryo development & Metabolic Analysis 206/232 (88. 7) 222/234 (94. 8) 95%CI of difference +1 – 11% P value 0. 02 Embs. with 100% blastomere survival (%) 106/206 (51. 4) 173/222 (77. 9) +18 - %35 Blastocyst formation (%) 102/206 (49. 5) 134/222 (60. 3) +1 – 20% 0. 02 Blasts. ≥ 3 AA (%) 43/102 (42. 1) 70/134 (52. 2) - 2. 7 – 22. 8% 0. 12 Hatching blastocysts (%) 22/102 (21. 5) 42/134 (31. 3) - 1. 4 – 20. 9% 0. 00 ) Cryosurvival (%) Vitrification Pyruvate uptake (pmol/embryo/h Slow Freezing 25 20 * 15 10 5 0 Slow Freezing Vitrification 0. 09 Balaban & Gardner et al. , HR 2008
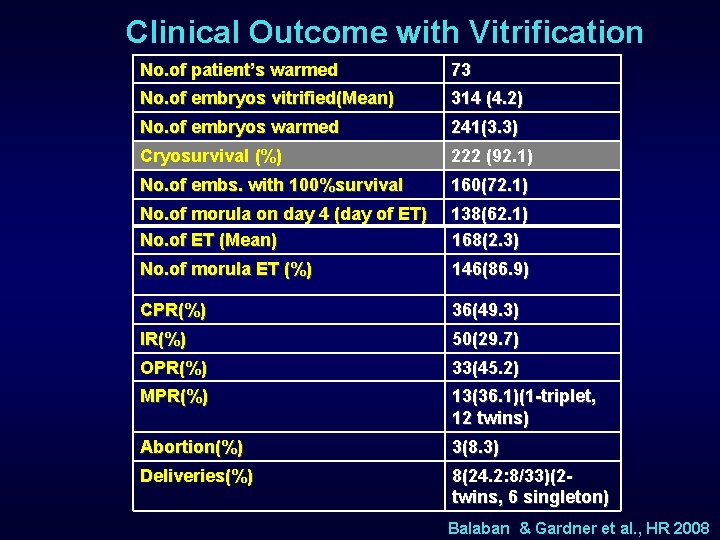
Clinical Outcome with Vitrification No. of patient’s warmed 73 No. of embryos vitrified(Mean) 314 (4. 2) No. of embryos warmed 241(3. 3) Cryosurvival (%) 222 (92. 1) No. of embs. with 100%survival 160(72. 1) No. of morula on day 4 (day of ET) No. of ET (Mean) 138(62. 1) 168(2. 3) No. of morula ET (%) 146(86. 9) CPR(%) 36(49. 3) IR(%) 50(29. 7) OPR(%) 33(45. 2) MPR(%) 13(36. 1)(1 -triplet, 12 twins) Abortion(%) 3(8. 3) Deliveries(%) 8(24. 2: 8/33)(2 twins, 6 singleton) Balaban & Gardner et al. , HR 2008

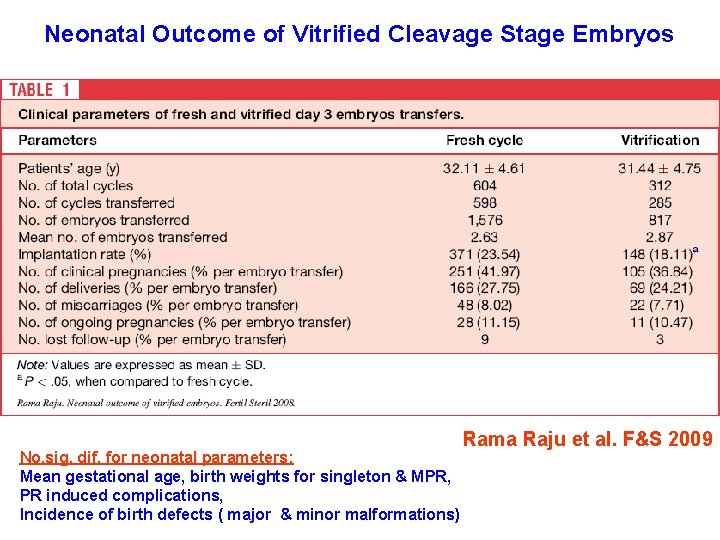
Neonatal Outcome of Vitrified Cleavage Stage Embryos No. sig. dif. for neonatal parameters: Mean gestational age, birth weights for singleton & MPR, PR induced complications, Incidence of birth defects ( major & minor malformations) Rama Raju et al. F&S 2009
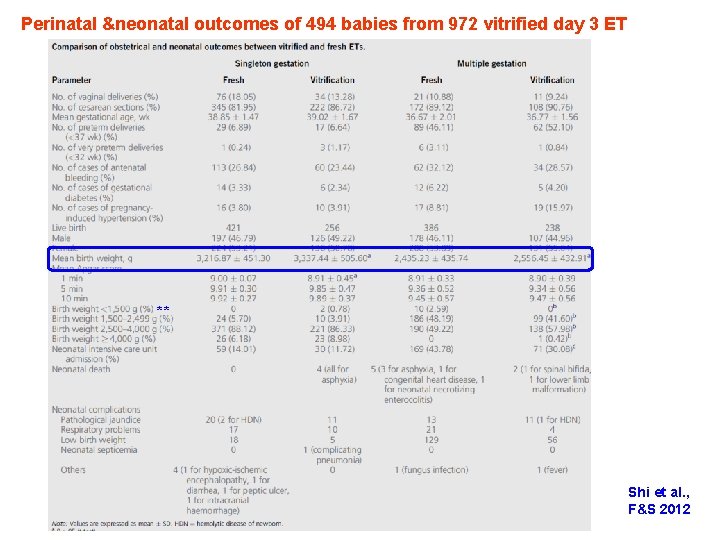
Perinatal &neonatal outcomes of 494 babies from 972 vitrified day 3 ET ** Shi et al. , F&S 2012
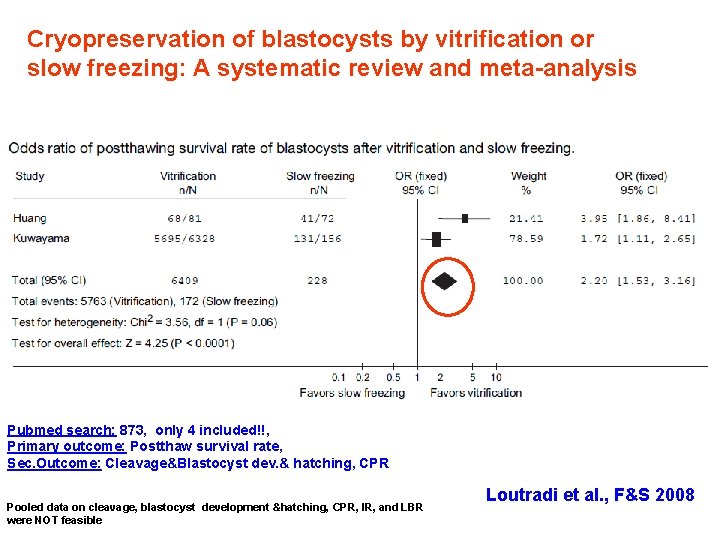
Cryopreservation of blastocysts by vitrification or slow freezing: A systematic review and meta-analysis Pubmed search: 873, only 4 included!!, Primary outcome: Postthaw survival rate, Sec. Outcome: Cleavage&Blastocyst dev. & hatching, CPR Pooled data on cleavage, blastocyst development &hatching, CPR, IR, and LBR were NOT feasible Loutradi et al. , F&S 2008
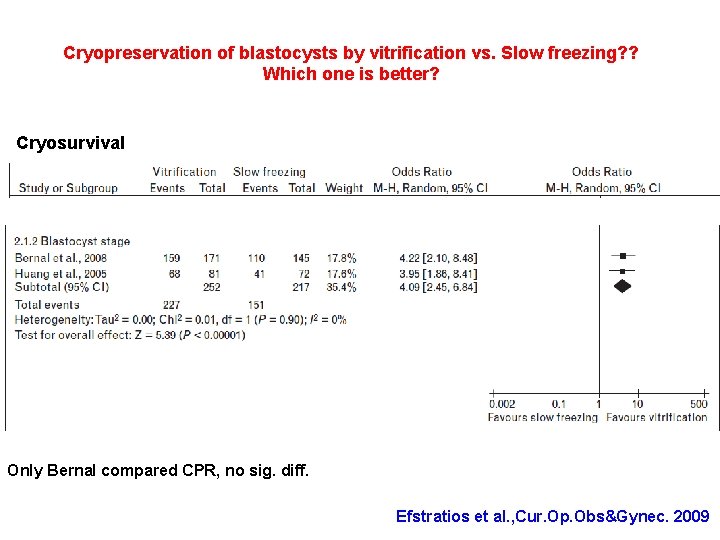
Cryopreservation of blastocysts by vitrification vs. Slow freezing? ? Which one is better? Cryosurvival Only Bernal compared CPR, no sig. diff. Efstratios et al. , Cur. Op. Obs&Gynec. 2009
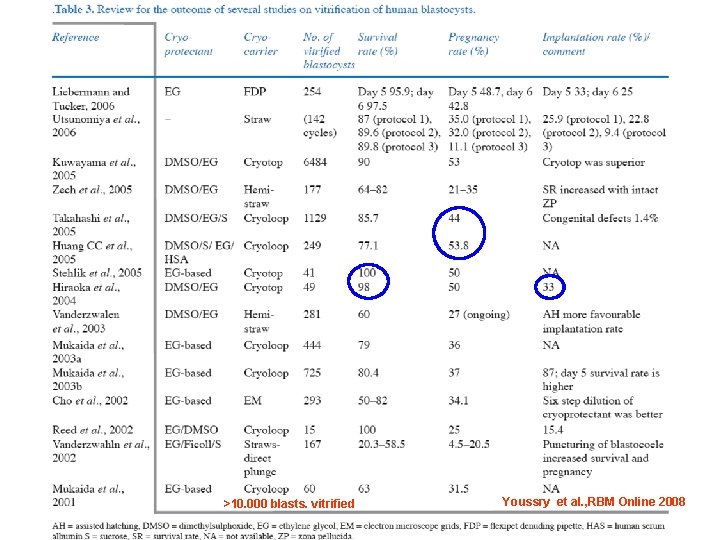
>10. 000 blasts. vitrified Youssry et al. , RBM Online 2008
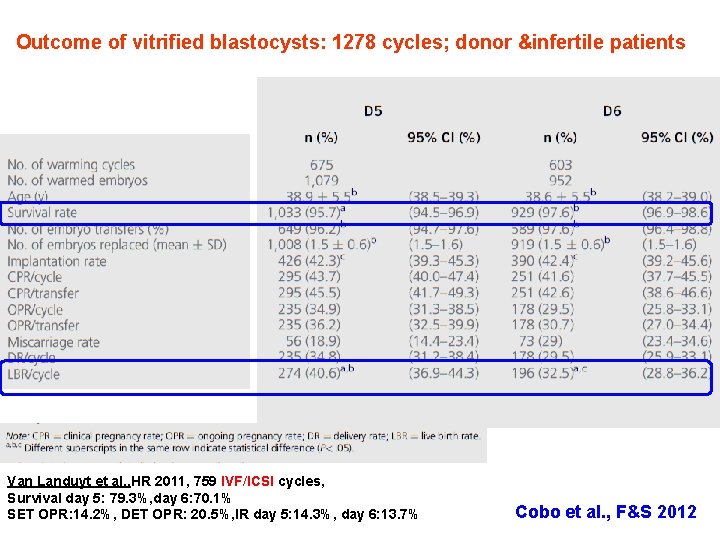
Outcome of vitrified blastocysts: 1278 cycles; donor &infertile patients Van Landuyt et al. , HR 2011, 759 IVF/ICSI cycles, Survival day 5: 79. 3%, day 6: 70. 1% SET OPR: 14. 2%, DET OPR: 20. 5%, IR day 5: 14. 3%, day 6: 13. 7% Cobo et al. , F&S 2012
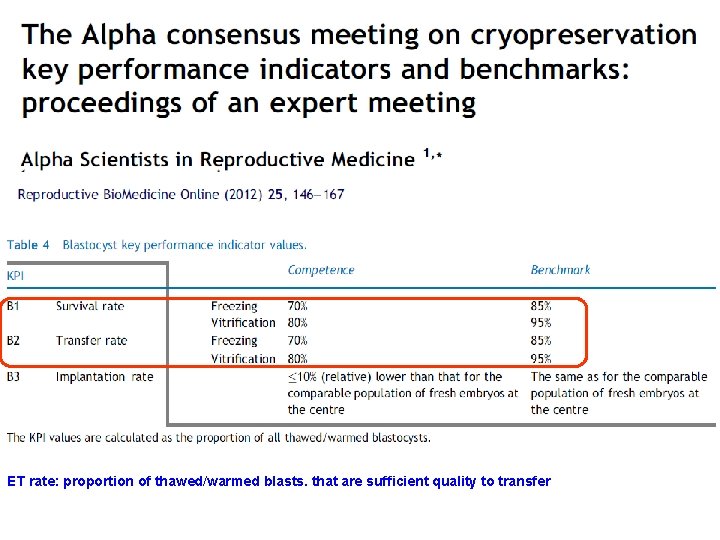
ET rate: proportion of thawed/warmed blasts. that are sufficient quality to transfer
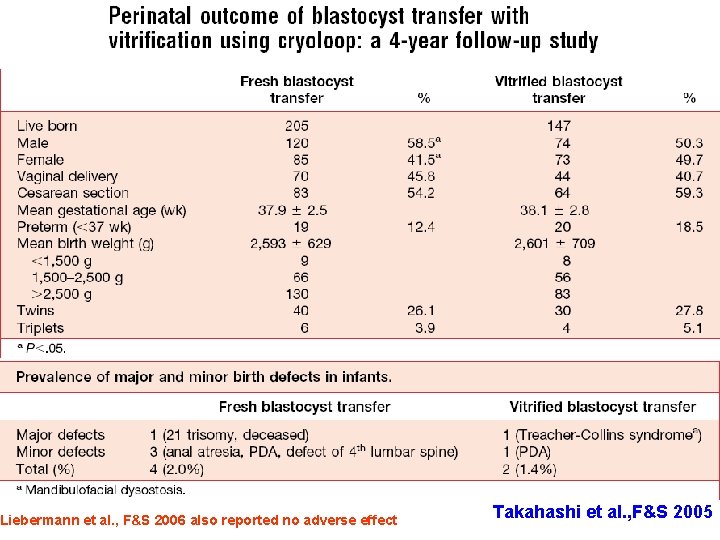
Liebermann et al. , F&S 2006 also reported no adverse effect Takahashi et al. , F&S 2005
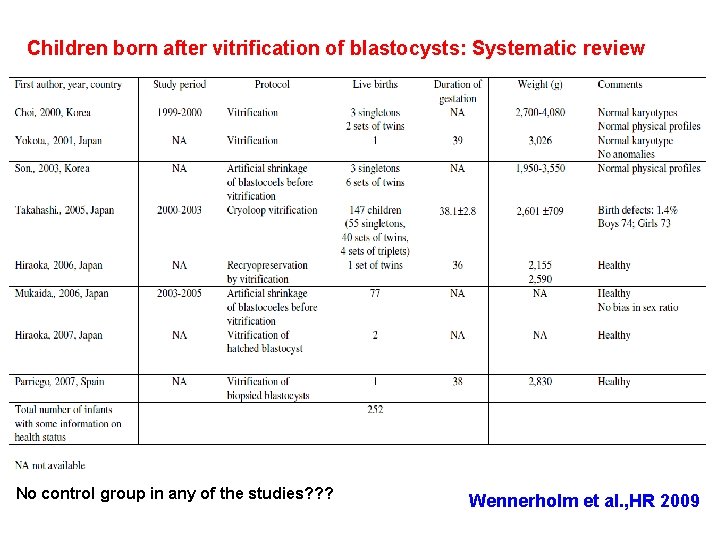
Children born after vitrification of blastocysts: Systematic review No control group in any of the studies? ? ? Wennerholm et al. , HR 2009
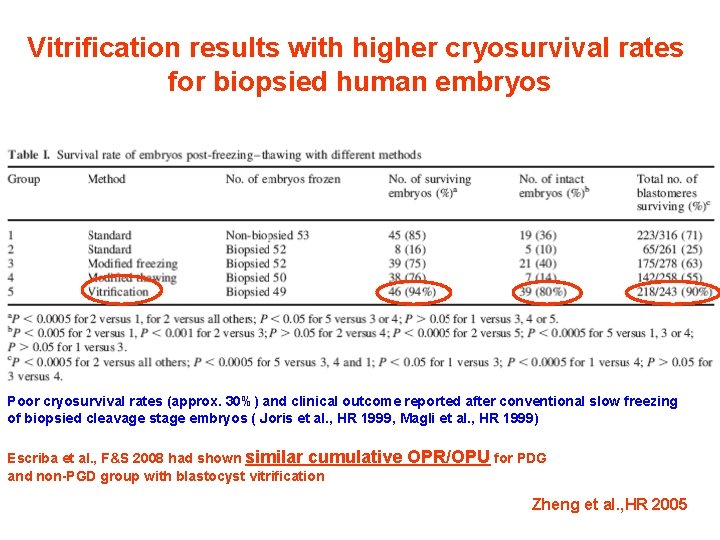
Vitrification results with higher cryosurvival rates for biopsied human embryos Poor cryosurvival rates (approx. 30%) and clinical outcome reported after conventional slow freezing of biopsied cleavage stage embryos ( Joris et al. , HR 1999, Magli et al. , HR 1999) Escriba et al. , F&S 2008 had shown similar cumulative and non-PGD group with blastocyst vitrification OPR/OPU for PDG Zheng et al. , HR 2005

Flexibility of re-cryopreserving cells by vitrification method • It’s presumed that refrozen & thawed embryos using conventional methods results with detrimental cryoinjury • • Chang C. RBM Online 2008 Two successful pregnancies obtained following oocyte vitrification and embryo re-vitrification. Kumasako et al. , F&S 2009 The efficacy of the transfer of twice frozen thawed embryos with the vitrification method Peng et al. , RBM Online 2011 Live birth after transfer of a twice vitrified warmed blastocyst that had undergone trophoectoderm biopsy James et al. , RBM Online 2012 Vitrification of human embryos previously cryostored by either slow freezing or vitrification results in high pregnancy rates

Concerns regarding Vitrification • Majority of the articles published on the clinical efficieny of vitrification for human cells utilized open carriers. And so LN 2 still remains to be a potential source of contamination since the technique is based on direct contact between the vitrification solution containing cryoprotectant agents and LN 2. So from a clinical point of view: *** Closed systems to avoid contamination are suggested, especially based on the new regulations of EUTCD. Few randomized clinical trials had shown similar efficiency. . (Kuwayama RBM 2005 -embryos, Van Landuyt HR 2011 -blasts. , Stoop RBM 2012 -oocytes) *** Storage of cells in the vapour phase of N 2 instead of LN 2(Cobo F&S 2010) *** Sterilization of LN 2 (Parmegiani RBM, HR 2011) • Safety of vitrification solutions with high concentrations of cryoprotectants? ? Low toxicity vitrification solutions must be designed in the future • Genetical structure of the vitrified cell? ? Chromosal abnormalities, gene expressions. . . More comparitive studies with fresh cells are needed to prove the safety of the technique

RESULTS • Vitrification protocols are now starting to enter the mainstream of human ART • Vitrification as a cryopreservation method has many primary advantages and benefits based on the published data • It’s likely that in near future vitrification will become the most suitable method for cryopreservation of any cells and perhaps tissues when concerns regarding safety are improved by scientifically properly designed studies
- Slides: 29