Vitalit cellulare proliferazione e funzionalit cellulare La sopravvivenza

































- Slides: 74


Vitalità cellulare, proliferazione e funzionalità cellulare La sopravvivenza cellulare, la proliferazione e altre importanti funzioni vitali - incluso apoptosi, adesione cellulare, chemiotassi, resistenza ad agenti farmacologici, endocitosi, secrezione e trasduzione del segnale - possono essere stimolate e monitorate con vari agenti chimici e reagenti biologici. Molti di questi processi inducono cambiamenti nella concentrazione di radicali liberi, di ioni o del potenziale di membrana che possono essere seguiti con indicatori fluorescenti appositi.

Vedremo rapidamente alcuni kit attualmente in commercio che permettono di valutare questi processi biologici. Ci occuperemo in particolare di kit per: 1. Saggi di proliferazione cellulare e sopravvivenza 2. Saggi di apoptosi 3. Saggi di adesione, chemiotassi e glutatione. Esistono anche sonde specifiche per valutare 1. Endocitosi ed esocitosi 2. Recettori di neurotrasmettitori 3. Canali ionici e trasportatori

1. Assays for Cell Viability, Cell Proliferation and Cell Cycle 1. 1 I saggi di sopravvivenza e citotossicità sono usati prevalentemente per valutare la proporzione di cellule vive e morte in una certa popolazione. Al contrario i saggi di proliferazione sono usati prevalentemente per monitorare la velocità di crescita di una popolazioe cellulare. Questi saggi basati sulla fluorescenza sono in generale meno rischiosi e meno costosi di tecniche radioisotopiche, più sensibili dei metodi colorimetrici e più convenienti dei test su animali.

In particolare i test fluorometrici di sopravvivenza e citotossicità sono facili da utilizzare con l’uso di un microscopio a fluorescenza, di un fluorimetro, di un lettore di micropiastra o di un citofluorimetro. Vedremo adesso reagenti appositamente studiati per saggi di sopravvivenza e citotossictà in una ampio spettro di cellule, incluse quelle di origine animale come pure lieviti e batteri. Per esempio vediamo il seguente kit: LIVE/DEAD Viability/Cytotoxicity Kit (sopravvivenza)

Principle of the Method (Molecular Probes) Il kit LIVE/DEAD® Viability/Cytotoxicity fornisce un test a due colori che è basato sulla determinazione simultanea delle cellule vive e di quelle morte utilizzando due sonde che misurano due parametri particolari: sopravvivenza — attività delle esterasi intracellulari e integrità della membrana cellulare Questo kit è anche usato per quantificare la morte cellulare per apoptosi e la citotossicità cellula-mediata

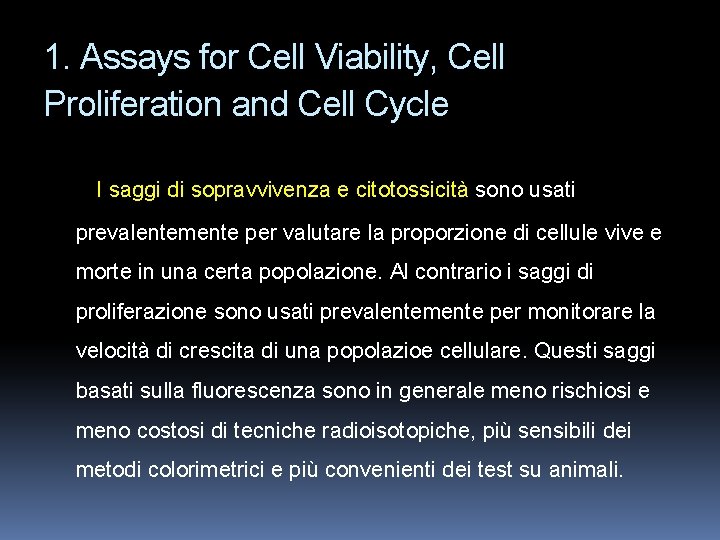
Le cellule vive si riconoscono per la presenza ubiquitaria di attività esterasica. Questa si valuta per conversione enzimatica di calceina AM (substrato delle esterasi) non fluorescente e permeabile attraverso la membrana in calceina fluorescente. Questa forma è ritenuta all’interno delle cellule vive che si colorano in verde. Eth. D-1 (etidio omodimero 1) entra invece nelle cellule con membrana danneggiata, si lega agli acidi nucleici e produce una luce rossa brillante (cellule morte). Eth. D-1 non pasa attraverso membrane cellulari sane.

Live and dead kangaroo rat (Pt. K 2) cells stained with ethidium homodimer-1 and the esterase substrate calcein AM

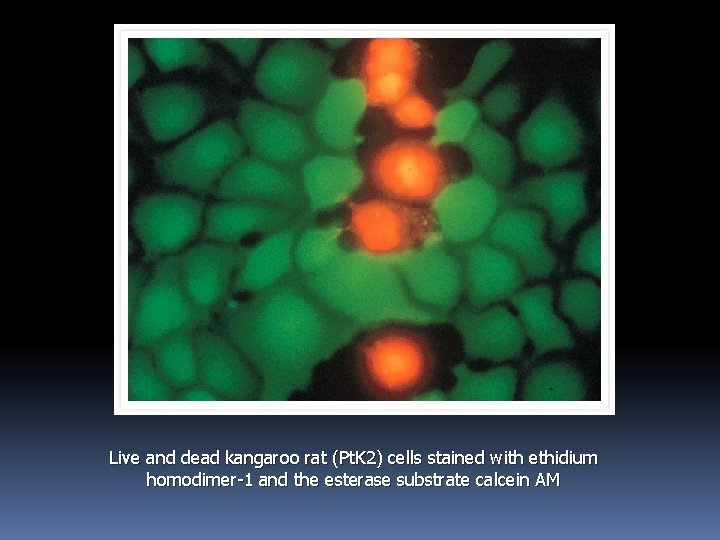
A mixed population of live and isopropyl alcohol–killed Micrococcus luteus and Bacillus cereus stained with the LIVE/DEAD Bac. Light Bacterial Viability Kit. Bacteria with intact cell membranes exhibit green fluorescence, whereas bacteria with damaged membranes exhibit red fluorescence.

La proliferazione cellulare e la caratterizzazione di sostanze in grado di promuovere o ritardare la proliferazione sono aree interessanti di studio per la biologia cellulare e la messa a punto di nuovi farmaci. Per vedere semplicemente le cellule e numerarle, le colorazioni fluorescenti che identificano le cellule in base a caratteristiche morfologiche, possono essere sufficienti. La particolare sensibilità di alcuni kit fluorescenti permette anche la quantificazione di alcuni virus.

Qualora si voglia invece valutare la proliferazione cellulare, **La variazione di quantità di DNA la cosa diviene più difficoltosa perché, attualmente, non vi *Brd. U è incorporato nelle cellule è associabile alle diverse fasi del sono dye fluorescenti che possano essere incamerati nella nuove e viene riconosciuto grazie ciclo cellulare. Il DNA è marcato cellula durante il processo mitotico. Per questo motivo la adpoi unse anticorpo che evidenzia con un fluoroforo specifico e maggior parte dei saggi di proliferazione stimano il numero quindi le cellule che proliferano. ne valuta la quantità. di cellule per incorporazione di un marcatore triziato, bromo deossi uridina (Brd. U, analogo della timidina)* nelle cellule, oppure misurando il contenuto totale di acidi nucleici** o di proteine nel lisato cellulare.

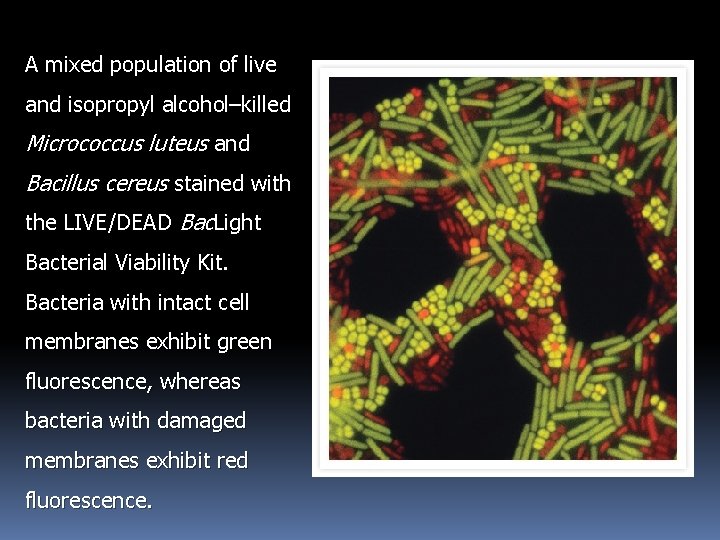
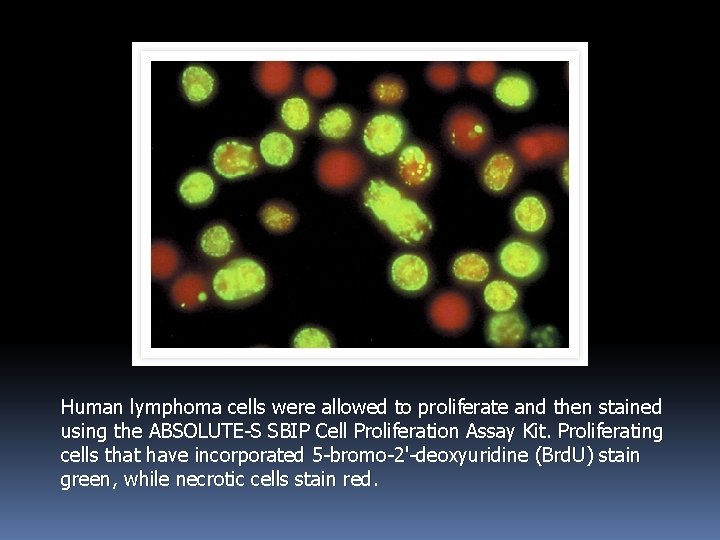
Human lymphoma cells were allowed to proliferate and then stained using the ABSOLUTE-S SBIP Cell Proliferation Assay Kit. Proliferating cells that have incorporated 5 -bromo-2'-deoxyuridine (Brd. U) stain green, while necrotic cells stain red

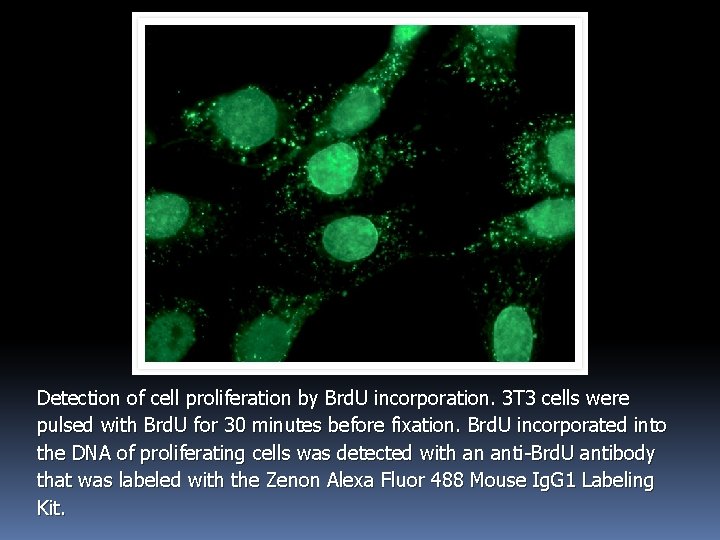
Detection of cell proliferation by Brd. U incorporation. 3 T 3 cells were pulsed with Brd. U for 30 minutes before fixation. Brd. U incorporated into the DNA of proliferating cells was detected with an anti-Brd. U antibody that was labeled with the Zenon Alexa Fluor 488 Mouse Ig. G 1 Labeling Kit.

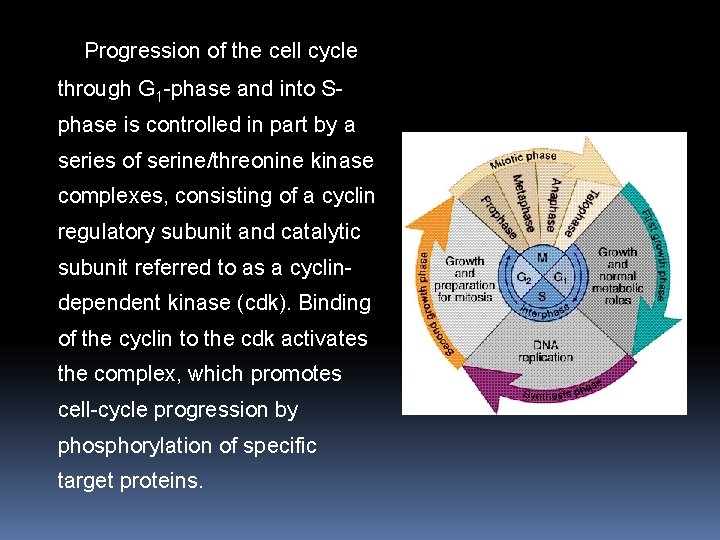
1. 3 Progression of the cell cycle through G 1 -phase and into Sphase is controlled in part by a series of serine/threonine kinase complexes, consisting of a cyclin regulatory subunit and catalytic subunit referred to as a cyclindependent kinase (cdk). Binding of the cyclin to the cdk activates the complex, which promotes cell-cycle progression by phosphorylation of specific target proteins.

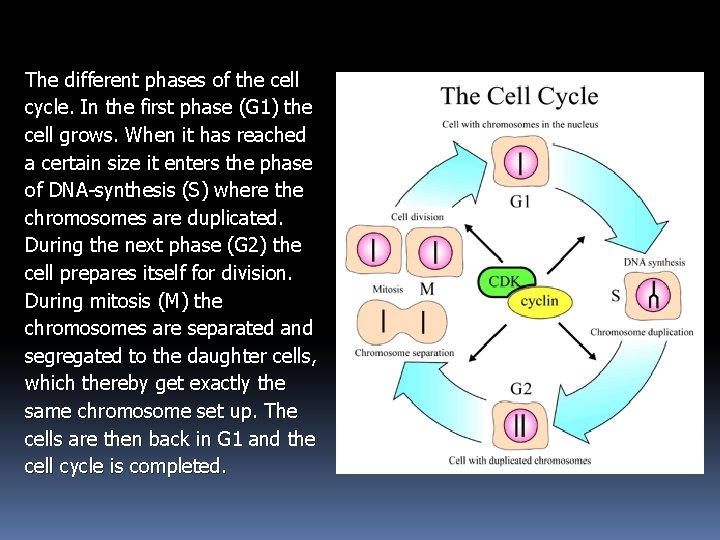
The different phases of the cell cycle. In the first phase (G 1) the cell grows. When it has reached a certain size it enters the phase of DNA-synthesis (S) where the chromosomes are duplicated. During the next phase (G 2) the cell prepares itself for division. During mitosis (M) the chromosomes are separated and segregated to the daughter cells, which thereby get exactly the same chromosome set up. The cells are then back in G 1 and the cell cycle is completed.

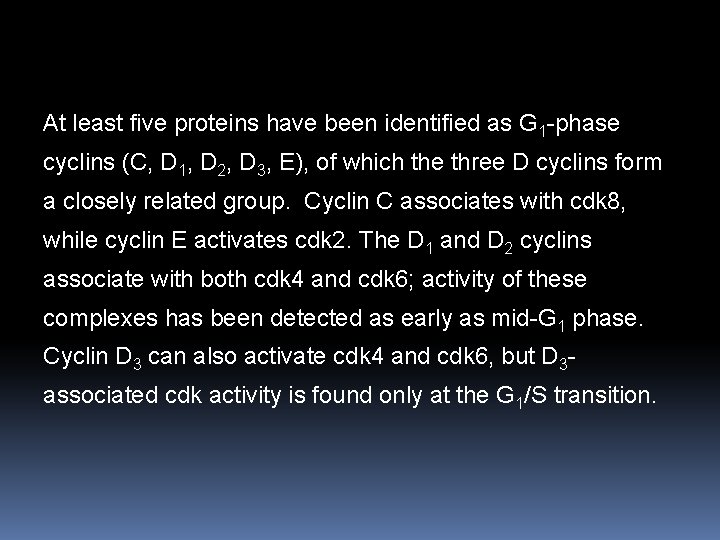
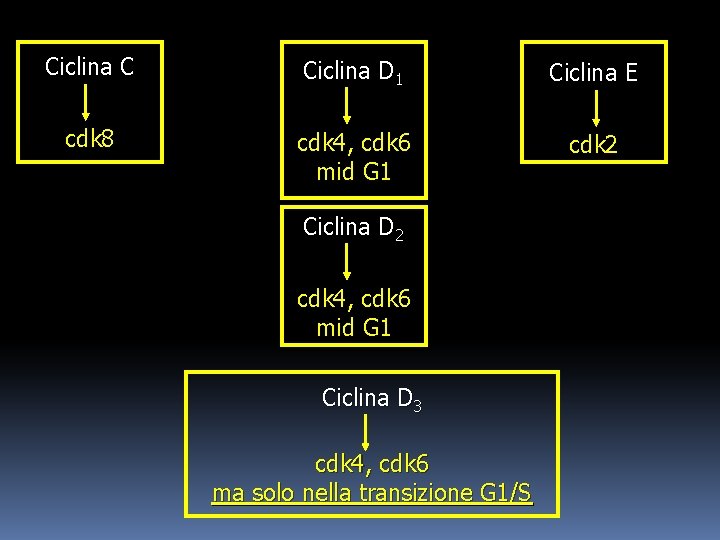
At least five proteins have been identified as G 1 -phase cyclins (C, D 1, D 2, D 3, E), of which the three D cyclins form a closely related group. Cyclin C associates with cdk 8, while cyclin E activates cdk 2. The D 1 and D 2 cyclins associate with both cdk 4 and cdk 6; activity of these complexes has been detected as early as mid-G 1 phase. Cyclin D 3 can also activate cdk 4 and cdk 6, but D 3 associated cdk activity is found only at the G 1/S transition.

Ciclina C Ciclina D 1 Ciclina E cdk 8 cdk 4, cdk 6 mid G 1 cdk 2 Ciclina D 2 cdk 4, cdk 6 mid G 1 Ciclina D 3 cdk 4, cdk 6 ma solo nella transizione G 1/S

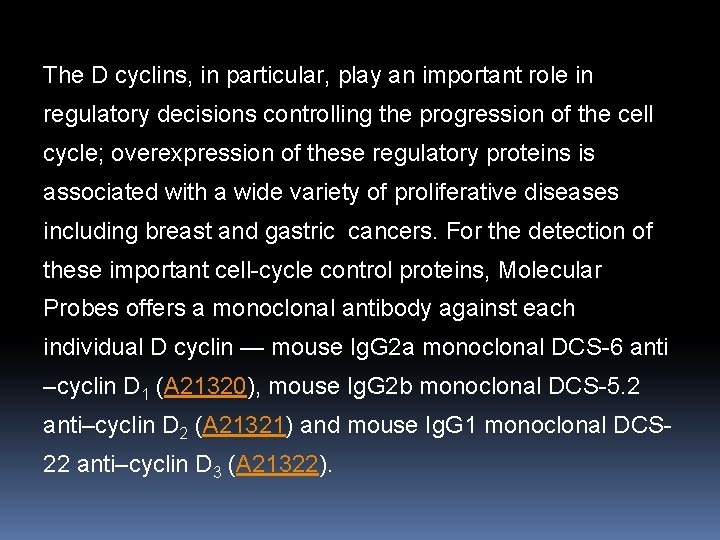
The D cyclins, in particular, play an important role in regulatory decisions controlling the progression of the cell cycle; overexpression of these regulatory proteins is associated with a wide variety of proliferative diseases including breast and gastric cancers. For the detection of these important cell-cycle control proteins, Molecular Probes offers a monoclonal antibody against each individual D cyclin — mouse Ig. G 2 a monoclonal DCS-6 anti –cyclin D 1 (A 21320), mouse Ig. G 2 b monoclonal DCS-5. 2 anti–cyclin D 2 (A 21321) and mouse Ig. G 1 monoclonal DCS 22 anti–cyclin D 3 (A 21322).

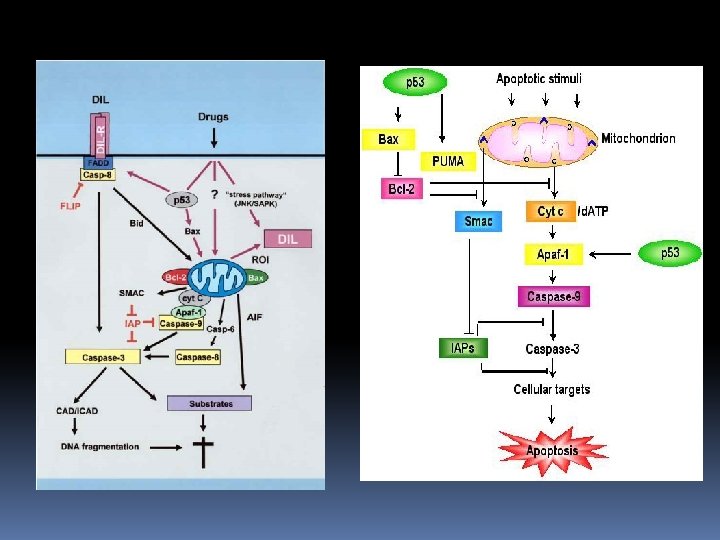
2. Apoptosis assay What is apoptosis? There are two ways in which cells die: they are killed by injurious agents. they are induced to commit suicide

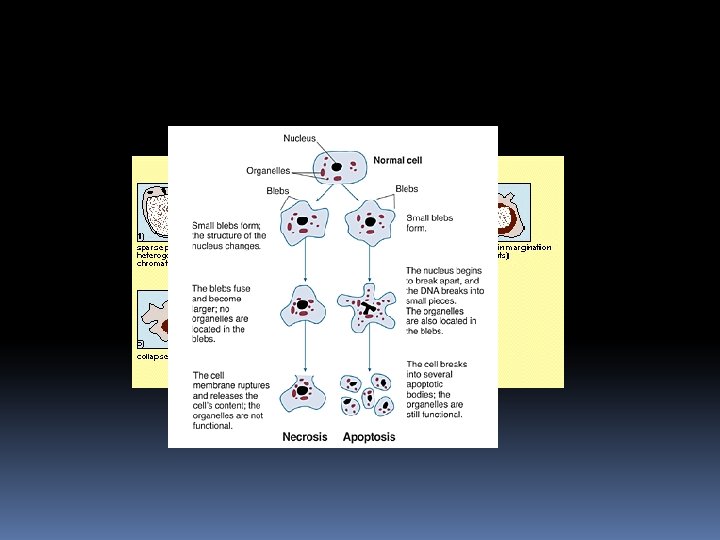
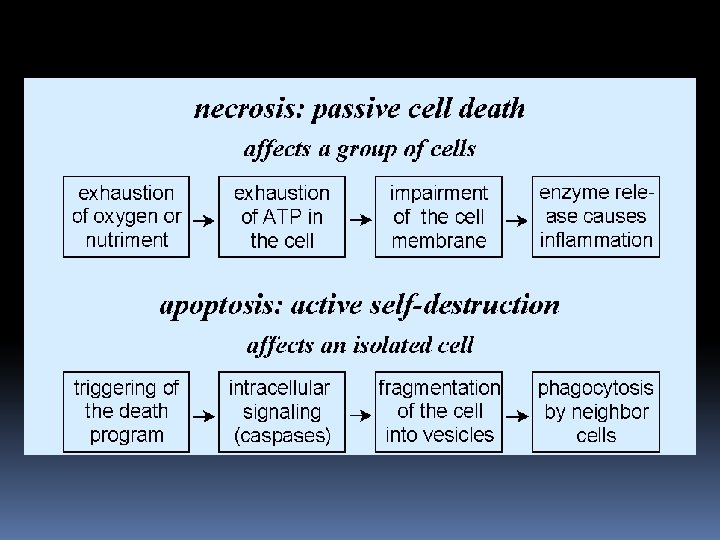
Cells that are damaged by injury, such as by mechanical damage or exposure to toxic chemicals undergo a characteristic series of changes: ü they (and their organelles like mitochondria) swell (because the ability of the plasma membrane to control the passage of ions and water is disrupted). ü the cell contents leak out, leading to inflammation of surrounding tissues.

Cells that are induced to commit suicide: Shrink and develop bubble-like blebs on their surface Have the chromatin (DNA and protein) in their nucleus degraded Their mitochondria break down with the release of cytochrome c Break into small, membrane-wrapped, fragments Phosphatidylserine, which is normally hidden within the plasma membrane, is exposed on the surface

Phosphatidylserine is bound by receptors on phagocytic cells like macrophages and dendritic cells which then engulf the cell fragments The phagocytic cells secrete cytokines that inhibit inflammation



The pattern of events in death by suicide is so orderly that the process is often called programmed cell death or PCD. The cellular machinery of programmed cell death turns out to be as intrinsic to the cell as, say, MITOSIS. Programmed cell death is also called apoptosis. Why should a cell commit suicide? There are two different reasons.

1. Programmed cell death is as needed for proper development as mitosis is. Examples: The formation of the fingers and toes of the fetus requires the removal, by apoptosis, of the tissue between them. The sloughing off of the inner lining of the uterus (the endometrium) at the start of menstruation occurs by apoptosis. The formation of the proper connections (synapses) between neurons in the brain requires that surplus cells be eliminated by apoptosis.

2. Programmed cell death is needed to destroy cells that represent a threat to the integrity of the organism. Examples Cells infected with viruses (cytotoxic T lymphocytes kill virus-infected cells by inducing apoptosis) Cells of the immune system (as cell-mediated immune responses wane, the effector cells must be removed to prevent them from attacking body constituents) Cells with DNA damage (cells respond to DNA damage by increasing their production of p 53 (p 53 is a potent inducer of apoptosis) Cancer cells

As with cell viability, no single parameter fully defines cell death in all systems; therefore, it is often advantageous to use several different approaches when studying apoptosis. Several methods have been developed to distinguish live cells from early and late apoptotic cells and from necrotic cells. Apoptotic cells are typically eliminated by phagocytosis; thus, apoptotic cells that have been selectively labeled with a fluorescent dye can potentially be used as tracers for phagocytosis.

Several kits have been developed to assay and detect apoptotic cells, based on the different molecular processes involved in the mechanism. The following is a list concerning these different ready-kits:

2. 1 DNA Stains for Detecting Apoptotic Cells The characteristic breakdown of the nucleus during apoptosis comprises collapse and fragmentation of the chromatin, degradation of the nuclear envelope and nuclear blebbing, resulting in the formation of micronuclei. Nucleic acid stains can be useful tools for identifying even low numbers of apoptotic cells in cell populations.

2. 2 Vybrant Apoptosis Assay Kit #4 detects apoptosis based on changes that occur in the permeability of cell membranes. This kit contains ready-to-use solutions of both the YOPRO-1 and propidium iodide nucleic acid stains. YO-PRO-1 nucleic acid stain selectively passes through the plasma membranes of apoptotic cells and labels them with moderate green fluorescence. Necrotic cells are stained with the red-fluorescent propidium iodide, a DNA-selective dye that is membrane impermeant but that easily passes through the compromised plasma membranes of necrotic cells.

2. 3. Vybrant Apoptosis Assay Kit #1 With the Vybrant Apoptosis Assay Kit #1 (V 13240), apoptotic cells are detected based on the externalization of phosphatidylserine.

2. 4. Apoptosis Assays Based on Protease Activity A distinctive feature of the early stages of apoptosis is the activation of caspase enzymes, the name applied to cysteine–aspartic acid specific proteases. Caspases are a key component of the apoptotic machinery of cells, participating in an enzyme cascade that results in cellular disassembly. The Vybrant FAM Caspase Assay Kits employ a novel approach for caspase detection that is based on a fluorescent caspase inhibitor (FLICA methodology).


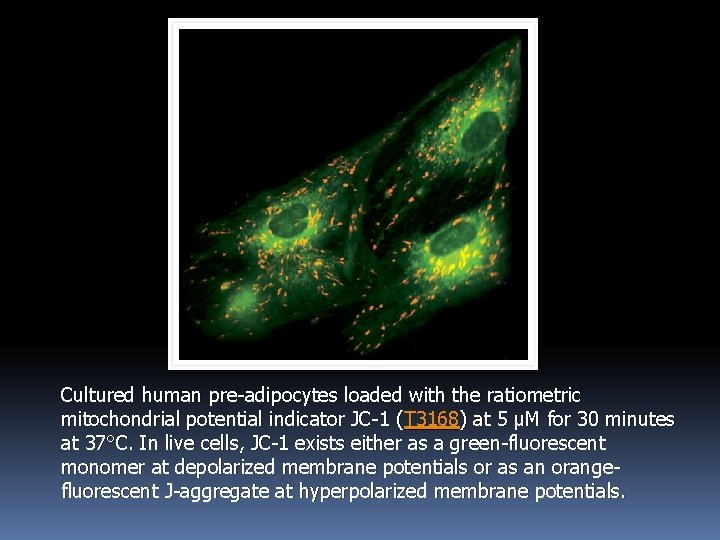
2. 5. Mitochondrial Stains for Detecting Apoptosis The green-fluorescent dye JC-1 exists as a monomer at low concentrations or at low membrane potential. However, at higher concentrations or at higher membrane potentials, JC-1 forms red-fluorescent "J-aggregates" that exhibit a broad excitation spectrum and an emission maximum at ~590 nm. Thus, the emission of this cyanine dye has been widely used to follow the changes in mitochondrial membrane potential that occur as a result of apoptosis.

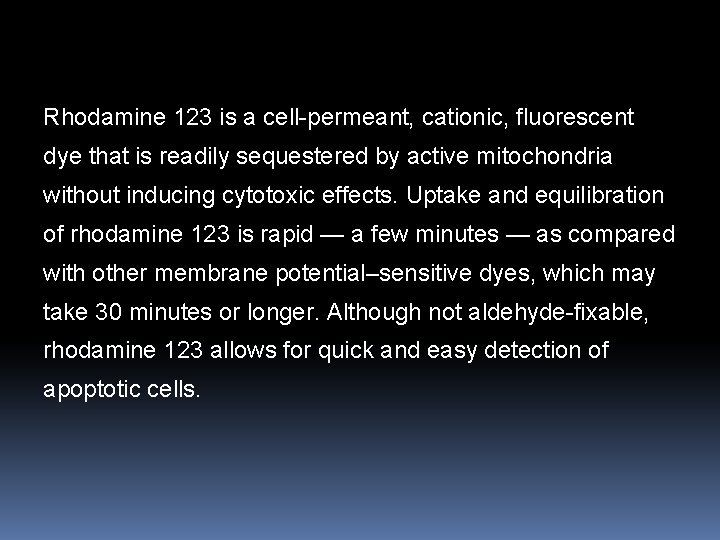
Rhodamine 123 is a cell-permeant, cationic, fluorescent dye that is readily sequestered by active mitochondria without inducing cytotoxic effects. Uptake and equilibration of rhodamine 123 is rapid — a few minutes — as compared with other membrane potential–sensitive dyes, which may take 30 minutes or longer. Although not aldehyde-fixable, rhodamine 123 allows for quick and easy detection of apoptotic cells.

Cultured human pre-adipocytes loaded with the ratiometric mitochondrial potential indicator JC-1 (T 3168) at 5 µM for 30 minutes at 37°C. In live cells, JC-1 exists either as a green-fluorescent monomer at depolarized membrane potentials or as an orangefluorescent J-aggregate at hyperpolarized membrane potentials.

3. 1 Cell Adhesion The fundamental role of cell–cell and cell–matrix adhesion in the morphology and development of organisms, organs and tissues has made identification of molecular mediators of cell adhesion an important research focus in cell biology. In a typical fluorescencebased cell adhesion assay, unlabeled cell monolayers in multiwell plates are incubated with fluorescently labeled cells and then washed to separate the adherent and nonadherent populations. Cell adhesion can then be determined simply by correlating the retained fluorescence with cell number.

An ideal fluorescent marker will retain proportionality between fluorescence and cell number and introduce minimal interference with the cell adhesion process. Because adhesion is a cellsurface phenomenon, cytoplasmic markers that can be passively loaded are preferable to compounds that label cell-surface molecules, provided they are retained in the cell for the duration of the experiment or their leakage rate can be independently measured. Adhesion of fluorescent dye–labeled cells to matrices such as bone an be directly observed by fluorescence microscopy using cells loaded with the permeant live-cell tracers.

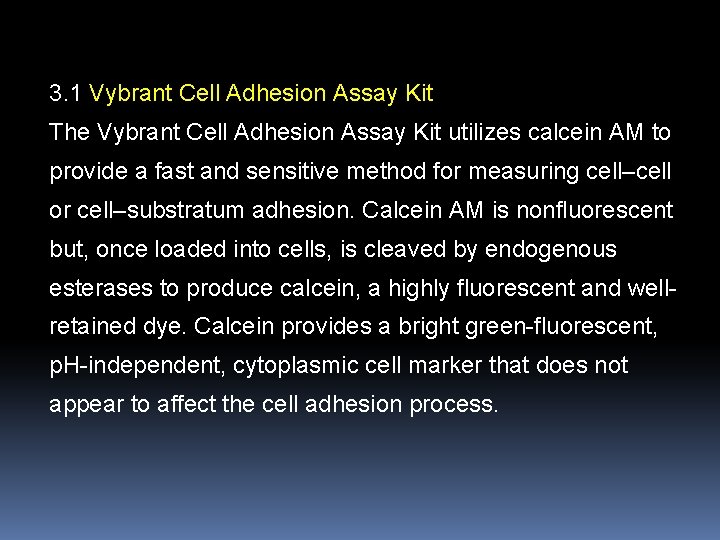
3. 1 Vybrant Cell Adhesion Assay Kit The Vybrant Cell Adhesion Assay Kit utilizes calcein AM to provide a fast and sensitive method for measuring cell–cell or cell–substratum adhesion. Calcein AM is nonfluorescent but, once loaded into cells, is cleaved by endogenous esterases to produce calcein, a highly fluorescent and wellretained dye. Calcein provides a bright green-fluorescent, p. H-independent, cytoplasmic cell marker that does not appear to affect the cell adhesion process.

3. 2 Fluorescent Gelatin and Type IV Collagen is a major component of the extracellular matrix and, in vertebrates, constitutes approximately 25% of total cellular protein. This important protein not only serves a structural role, but also is important in cell adhesion and migration. Specific collagen receptors, fibronectin and a number of other proteins involved in cell–cell and cell– surface adhesion have been demonstrated to bind collagen and gelatin (denatured collagen). Molecular Probes prepares fluorescent conjugates of gelatin and type IV collagen the principal collagen in basement membranes.

3. 3 Anti-Fibronectin Antibody Fibronectin is a large glycoprotein that is found in both plasma and in the extracellular matrix. The protein is coded by a single gene, but alternate RNA splicing gives rise to several fibronectin isoforms that play important roles in cellular adhesion and migration, blood clotting and phagocytosis. The apparent function of fibronectin is to mediate cell attachment via interactions with both cellsurface receptors and components of the extracellular matrix such as heparin, fibrinogen and collagen.

Molecular Probes offers a chicken Ig. Y anti-fibronectin (human) antibody (A 21316, Anti-Fibronectin (Human) Chicken Ig. Y Fraction) for detecting fibronectin in Western blotting and immunohistochemistry applications, as well as several fluorescent anti–chicken Ig. G secondary antibodies that recognize this antibody.

3. 2 Chemotaxis Assays Chemotaxis, defined as directed cell motion toward an extracellular gradient, plays an important role during fertilization, inflammation, wound healing and hematopoiesis. Measurement of chemotaxis usually has relied on determination of the number of viable cells that have migrated through a special "chemotaxis chamber. " The probes used to follow chemotaxis in live cells typically are the same esterase substrates that are useful for assaying cell viability and cell adhesion.

The primary esterase substrates used for this purpose are calcein AM (C 1430, C 3099, C 3100 MP), BCECF AM (B 1150, B 1170, B 3051) and Cell. Tracker Green CMFDA (C 2925, C 7025). Calcein AM does not interfere with lymphocyte proliferation or with granulocyte or neutrophil chemotaxis or superoxide production, and, unlike BCECF AM, calcein AM does not affect chemotaxis in leukocytes.

3. 3 Glutathione Determination The tripeptide glutathione ( -L-glutamyl-L-cysteinylglycine) is the most abundant (up to 10 m. M in the cytoplasm and about 1 m. M in blood) and important non-protein thiol in mammalian cells. Glutathione plays a central role in protecting cells of all organs, including the brain, against damage produced by free radicals, oxidants and electrophiles. Several fluorescent reagents have been proposed for determining cellular levels of glutathione and glutathione S-transferase (GST), which catalyzes the formation of glutathione S-conjugates, but no probe is without drawbacks in quantitative studies of live cells.

The high but variable levels of intracellular glutathione and the multitude of GST isozymes make kinetic measurements under saturating substrate conditions difficult or impossible. Isozymes of GST vary both in abundance and activity, further complicating the analysis. Moreover, the fluorescent reagents designed to measure glutathione may react with intracellular thiols other than glutathione, including proteins in glutathione-depleted cells.

Therefore, precautions must be taken in applying the reagents mentioned here to quantitate either glutathione or GST in cells. A useful strategy is to test a variety of glutathione-sensitive dyes — those requiring glutathione Stransferase activity, as well as GST-independent fluorophores — under controlled experimental conditions in which glutathione is depleted.

3. 3. 1 Glutathione Determination with Monochlorobimane Cell-permeant monochlorobimane (m. BCl, M 1381 MP), which is essentially nonfluorescent until conjugated to thiols, has long been the preferred thiol-reactive probe for quantitating glutathione levels in cells and for measuring GST activity. Because the blue-fluorescent glutathione adduct of monochlorobimane eventually accumulates in the nucleus, it is not a reliable indicator of the nuclear and cytoplasmic distribution of cellular glutathione. Tissue glutathione levels can also be measured fluorometrically by adding both monochlorobimane and glutathione Stransferase to tissue homogenates

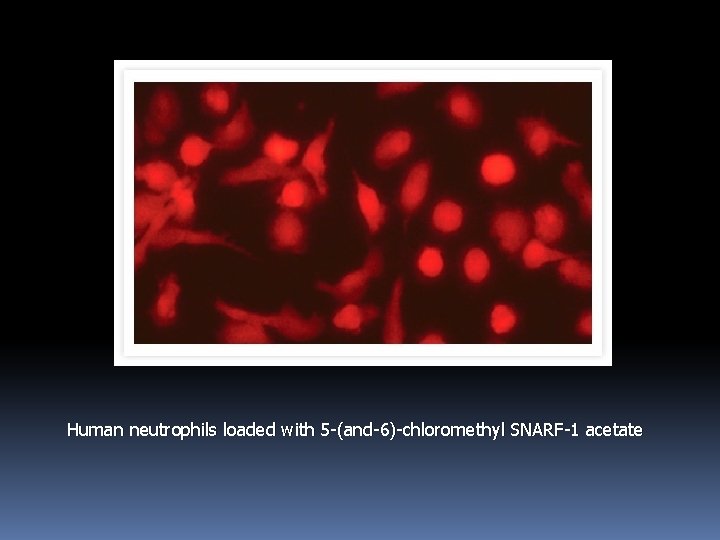
3. 3. 2 Glutathione Determination with Visible Light–Excitable Thiol-Reactive Probes Cell. Tracker Green CMFDA (5 -chloromethylfluorescein diacetate, C 2925, C 7025) is a useful alternative to the UV light–excitable monochlorobimane for determining levels of intracellular glutathione. Cell. Tracker Green CMFDA can be excited by the argon-ion laser. Like CMFDA, chloromethyl SNARF-1 acetate (C 6826) forms adducts with intracellular thiols that are well retained by viable cell.

Human neutrophils loaded with 5 -(and-6)-chloromethyl SNARF-1 acetate

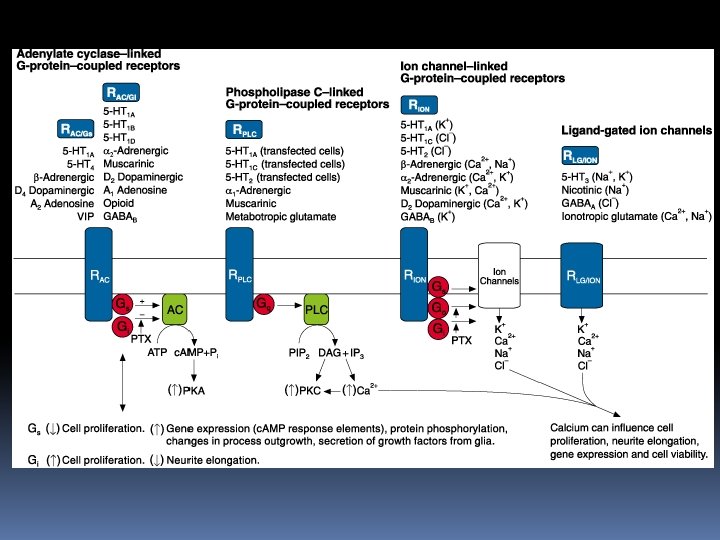
Probes for Neurotransmitter Receptors Because receptor-mediated signal transduction underlies much of what occurs in cellular biochemistry and physiology, fluorescent receptor ligands can provide a sensitive means of identifying and localizing some of the most pivotal molecules in cell biology. Molecular Probes offers fluorescently labeled and unlabeled ligands for various cellular receptors, ion channels and ion carriers. Many of these site-selective fluorescent probes may be used on live or fixed cells, as well as in cell-free extracts.

In particular, we would like to highlight those ligands conjugated to the green-fluorescent Alexa Fluor 488, BODIPY FL and Oregon Green 514 dyes and the redfluorescent Alexa Fluor 594 and Texas Red dyes, which provide extremely bright signals and superior photostability. The high sensitivity and selectivity of these fluorescent probes make them especially good candidates for measuring low-abundance receptors

Un esempio: α-Bungarotoxin Probes for Nicotinic Acetylcholine Receptors Nicotinic acetylcholine receptors (nicotinic ACh. Rs) are neurotransmitter-gated ion channels that produce an increase in Na+ and K+ permeability, depolarization and excitation upon activation by acetylcholine. α -Bungarotoxin, a 74–amino acid peptide extracted from Bungarus multicinctus venom, binds with high affinity to the α -subunit of the nicotinic ACh. R of neuromuscular junctions.


Molecular Probes provides an extensive selection of fluorescent α -bungarotoxin conjugates (Table 16. 4, alpha. Bungarotoxin and Conjugates) to facilitate visualization of nicotinic ACh. Rs with a variety of instrumentation. We attach approximately one fluorophore to each molecule of α bungarotoxin, thus retaining optimal binding specificity. The labeled bungarotoxins are then chromatographically separated from unlabeled molecules to ensure adequate labeling of the product.

Fluorescent α-bungarotoxins have been used in a variety of informative investigations of the nicotinic ACh. R to: • Correlate receptor clustering during neuromuscular development with tyrosine phosphorylation of the receptor. • Document nicotinic ACh. R cluster formation after myoblast fusion. • Quantitate nicotinic ACh. Rs in a study that showed that several isotypes of agrin, a component of synaptic basal lamina, help trigger the nicotinic ACh. R aggregation that occurs during neuromuscular junction formation. • Detect reinnervation of adult muscle after nerve damage and to identify and visualize endplates.

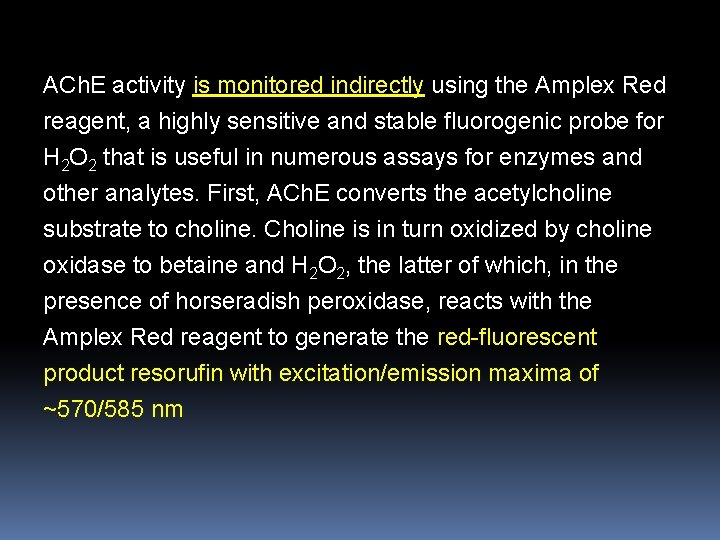
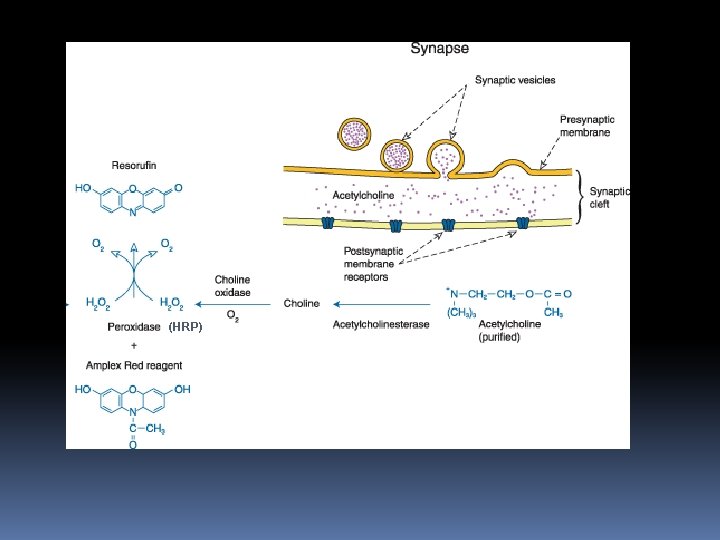
Amplex Red Acetylcholine/Acetylcholinesterase Assay Kit The action of acetylcholine (ACh) at neuromuscular junctions is regulated by acetylcholinesterase (ACh. E), the enzyme that hydrolyzes ACh to choline and acetate. The Amplex Red Acetylcholine/Acetylcholinesterase Assay Kit provides an ultrasensitive method for continuously monitoring ACh. E activity and for detecting Ach. Other potential uses for this kit include screening for ACh. E inhibitors and measuring the release of ACh from synaptosomes.

ACh. E activity is monitored indirectly using the Amplex Red reagent, a highly sensitive and stable fluorogenic probe for H 2 O 2 that is useful in numerous assays for enzymes and other analytes. First, ACh. E converts the acetylcholine substrate to choline. Choline is in turn oxidized by choline oxidase to betaine and H 2 O 2, the latter of which, in the presence of horseradish peroxidase, reacts with the Amplex Red reagent to generate the red-fluorescent product resorufin with excitation/emission maxima of ~570/585 nm

(HRP)

Per altri neurotrasmettitori Pirenzepine Probes for Muscarinic Acetylcholine Receptors: Unlike nicotinic ACh. Rs, muscarinic acetylcholine receptors (muscarinic ACh. Rs) are G-protein–coupled receptors that produce either excitatory or inhibitory responses and are not necessarily associated with changes in ion permeability. Fluorescent derivatives of pirenzepine are selective antagonists for the M 1 muscarinic ACh. R. The green-fluorescent BODIPY FL and red-fluorescent BODIPY 558/568 derivatives retain pirenzepine's specificity for the M 1 muscarinic receptor and exhibit similar inhibition and displacement profiles.

Prazosin Probes for α 1 -Adrenergic Receptors: the greenfluorescent BODIPY FL and red-fluorescent BODIPY 558/568 derivatives of prazosin, a high-affinity antagonist for the α 1 adrenergic receptor are available. These derivatives can be used to localize the α 1 -adrenergic receptors on cultured cortical neurons. BODIPY TMR-X Muscimol for the GABAA Receptor: Muscimol is a powerful agonist of the GABAA receptor and has been widely used to reversibly inactivate localized groups of neurons. With the introduction of the BODIPY TMR-X muscimol conjugate researchers can avoid using radioactive methods to map the distribution of the drug in the central nervous system, as well as to detect the presence of GABAA receptors on cell surfaces.

Neuropeptide Probes for the Neurokinin Receptors: Substance P is an agonist of the neurokinin 1 (NK 1) receptor, a member of the seven -domain transmembrane GPCRs. Substance P is a tachykinin — one of a group of neuropeptides that are primarily involved in the mediation of inflammatory responses. NK 1 receptor probes include: • Alexa Fluor 488 and Oregon Green 488 conjugates of substance P: the Alexa Fluor 488 and Oregon Green 488 dyes virtually match fluorescein's excitation and emission spectra but have the additional benefits of superior photostability and lower p. H sensitivity. • Fluorescein conjugate of substance P • Tetramethylrhodamine conjugate of substance P : substance P labeled with the photostable, orange-fluorescent tetramethylrhodamine dye offers researchers another option for multicolor experiments.

Anti–NMDA Receptor Antibodies: NMDA receptors constitute cation channels of the CNS that are gated by the excitatory neurotransmitter L-glutamate. Activation of NMDA receptors is essential for inducing LTP, a form of activity-dependent synaptic plasticity that is implicated in the learning process in animal behavioral models. The biophysical properties of NMDA receptor channels contributing to LTP include Ca 2+ permeability, voltage-dependent Mg 2+ blocking and slow-gating kinetics. NMDA receptor–channel activities play a role in neuronal development and in disorders such as epilepsy and ischemic neuronal cell death.

In vitro reconstitution experiments with the cloned NMDA receptor subunit 1 and any one of the four NMDA receptor subunits 2 A, 2 B, 2 C and 2 D revealed that the physical properties of the heteromeric NMDA receptor channel appear to be imparted by the particular NMDA receptor subunit 2. NMDA receptor subunits 2 A and 2 B are detected predominantly in the hippocampus and cortex, whereas 2 C is found mainly in the cerebellum. Thus, cellular expression profiles of the NMDA receptor subunits 2 A, 2 B, 2 C and 2 D may contribute to the biophysical properties of NMDA receptors in specific central neurons.

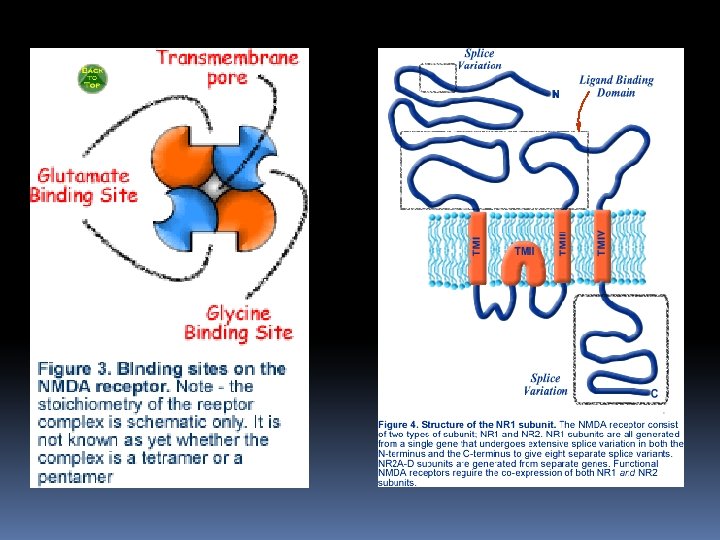
NMDA receptor overview NMDA receptors are composed of assemblies of NR 1 subunits and NR 2 subunits, which can be one of four separate gene products (NR 2 A-D). Expression of both subunits are required to form functional channels. The glutamate binding domain is formed at the junction of NR 1 and NR 2 subunits (hence the need for both subunits to be expressed). In addition to glutamate, the NMDA receptor requires a co-agonist, glycine, to bind to allow the receptor to function. The glycine binding site is found on the NR 1 subunit. The NR 2 B subunit also has a binding site for ployamines, regulatory molecules that modulate the functioning of the NMDA receptor.


There affinity-purified rabbit polyclonal antibodies to NMDA receptor subunits 2 A, 2 B and 2 C. The anti–NMDA receptor subunit 2 A and 2 B antibodies were generated against fusion proteins containing amino acid residues 1253– 1391 of subunit 2 A and 984– 1104 of subunit 2 B, respectively. These two antibodies are active against mouse, rat and human forms of the antigens and are specific for the subunit against which they were generated. In contrast, the anti–NMDA receptor subunit 2 C antibody was generated against amino acid residues 25– 130 of subunit 2 C and recognizes the 140, 000 -dalton subunit 2 C, as well as the 180, 000 dalton subunit 2 A and subunit 2 B from mouse, rat and human. These three affinity-purified antibodies are suitable for immunohistochemistry, Western blots, enzyme-linked immunosorbent assays (ELISAs) and immunoprecipitations.

Probes for Ion Channels and Carriers Probes for Ca 2+ Channels and Carriers In both excitable and nonexcitable cells, intracellular Ca 2+ levels modulate a multitude of vital cellular processes — including gene expression, cell viability, cell proliferation, cell motility and cell shape and volume regulation — thereby playing a key role in regulating cell responses to external activating agents. These dynamic changes in intracellular Ca 2+ levels are regulated by ligand-gated and G-protein–coupled ion channels in the plasma membrane, as well as by mobilization of Ca 2+ from intracellular stores.

One of the best-studied examples of Ca 2+-dependent signal transduction is the depolarization of excitable cells, such as those of neuronal, cardiac, skeletal and smooth muscle tissue, which is mediated by inward Ca 2+ and Na+ currents. The Ca 2+ current is attributed to the movement of ions through N-, L-, P- and T-type Ca 2+ channels, which are defined both pharmacologically and by their biophysical properties, including conductance and voltage sensitivity. Molecular Probes offers fluorescent analogs of dihydropyridine and verapamil as ligands for N- and L-type Ca 2+ channels. In addition, exist unlabeled and fluorescent derivatives of ryanodine, a powerful modulator of the intracellular Ca 2+ channels found in the sarcoplasmic reticulum and other subcellular organelles.

Fluorescent Dihydropyridines for L-Type Ca 2+ Channels: The Ltype Ca 2+ channel is readily blocked by the binding of dihydropyridines to the channel's pore-forming α 1 -subunit. The high-affinity (–)-enantiomer of dihydropyridine is available labeled with either the green-fluorescent DM-BODIPY or the orangefluorescent ST-BODIPY fluorophore. DM-BODIPY dihydropyridine has been employed to investigate the molecular mechanism for dihydropyridine binding to L-type channels.

Ryanodine Probes for Intracellular Ca 2+ Channels: Ryanodine is a plant alkaloid that mobilizes Ca 2+ from intracellular stores by activating a class of Ins 1, 4, 5 -P 3–insensitive Ca 2+ channels. It alters the function of the Ca 2+ channel in a complex manner: submicromolar concentrations lock the channel in a long-lived open state, whereas micromolar or greater concentrations inhibit Ca 2+ release. BODIPY FL-X ryanodine has been used to visualize ryanodine receptor distribution in live porcine endothelial cells, in pancreatic β-cells, in vascular myocytes, in cardiomyocytes, in frog sympathetic neurons and in the rat parotid gland.

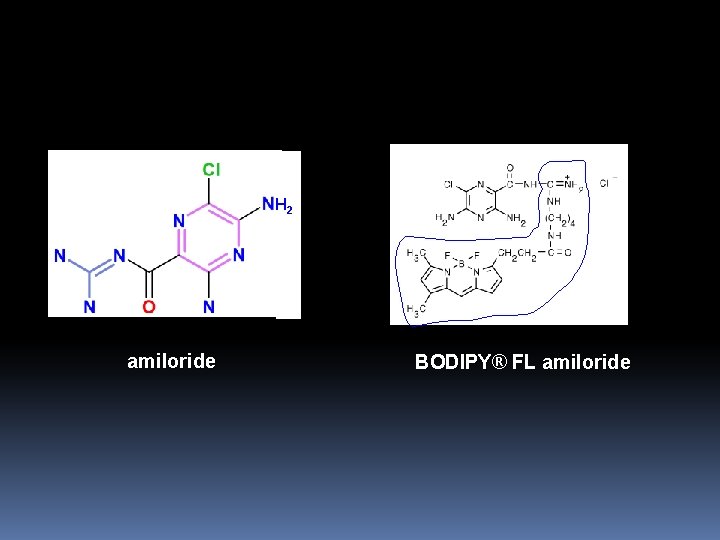
Probes for Na+ Channels and Carriers Amiloride Analogs: Probes for the Na+ Channel and the Na+/H+ Antiporter: Amiloride is a compound known to inhibit the Na+/H+ antiport of vertebrate cells by acting competitively at the Na+binding site. The antiport extrudes protons from cells using the inward Na+ gradient as a driving force. More than 1000 different amiloride analogs have been synthesized and many of these tested for their specificity and potency in inhibiting the Na+ channel, Na+/H+ antiporter and Na+/Ca 2+ exchanger. We offer BODIPY FL amiloride a green-fluorescent probe in which the BODIPY fluorophore is attached at the R 3 position.

H 2 amiloride BODIPY® FL amiloride