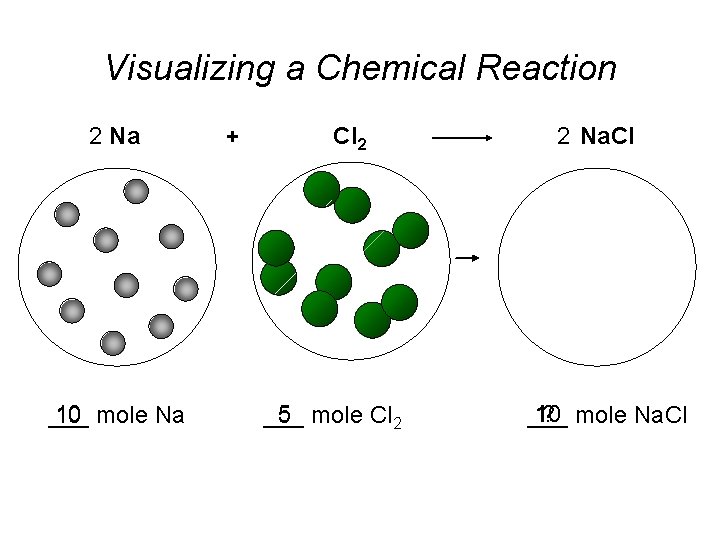
Visualizing a Chemical Reaction 2 Na 10 mole

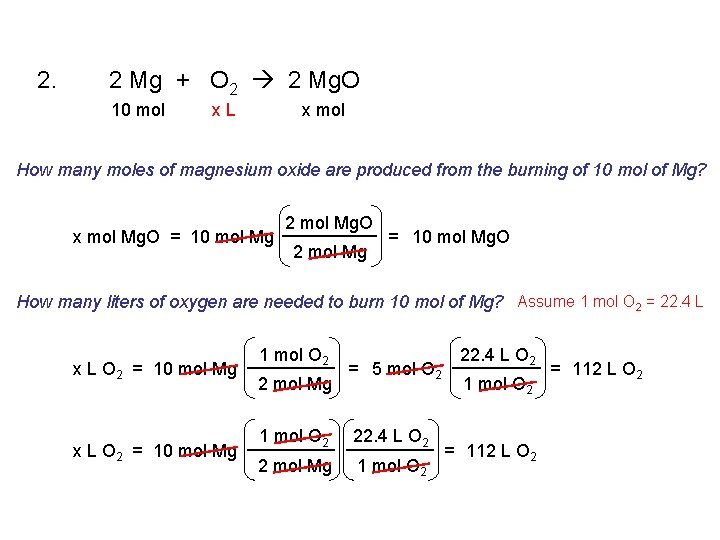
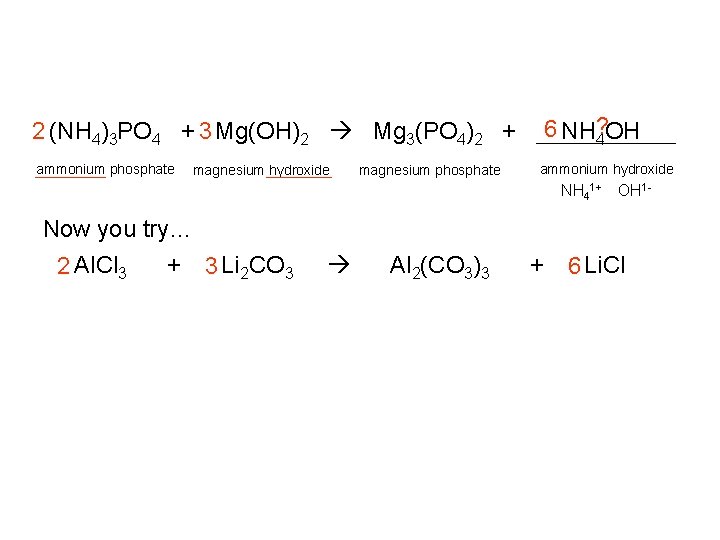

- Slides: 24

Visualizing a Chemical Reaction 2 Na 10 mole Na ___ + Cl 2 5 mole Cl 2 ___ 2 Na. Cl 10 ? mole Na. Cl ___

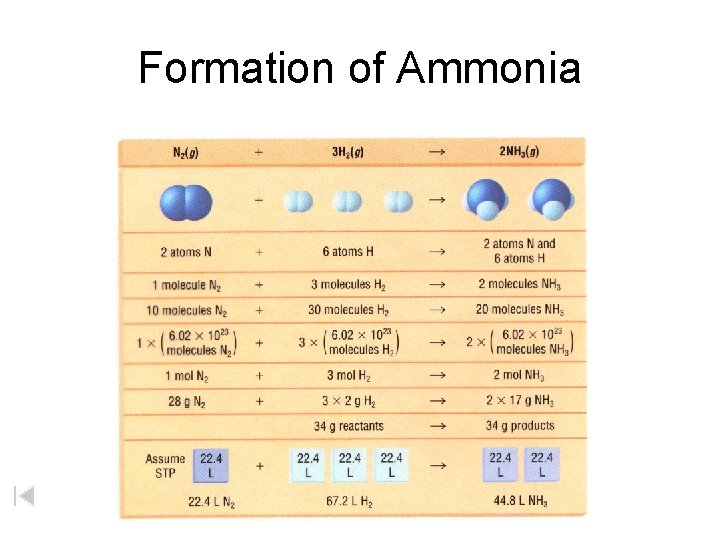
Formation of Ammonia

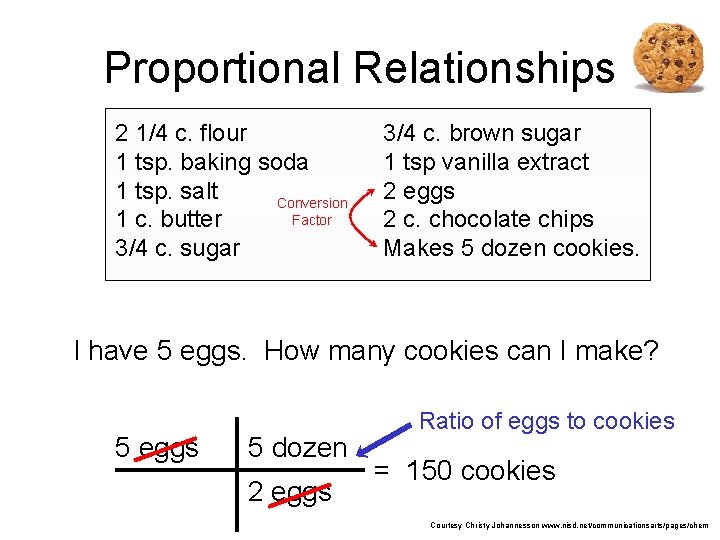
Proportional Relationships 2 1/4 c. flour 1 tsp. baking soda 1 tsp. salt Conversion Factor 1 c. butter 3/4 c. sugar 3/4 c. brown sugar 1 tsp vanilla extract 2 eggs 2 c. chocolate chips Makes 5 dozen cookies. I have 5 eggs. How many cookies can I make? 5 eggs 5 dozen 2 eggs Ratio of eggs to cookies 150 dozen cookies = 12. 5 Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

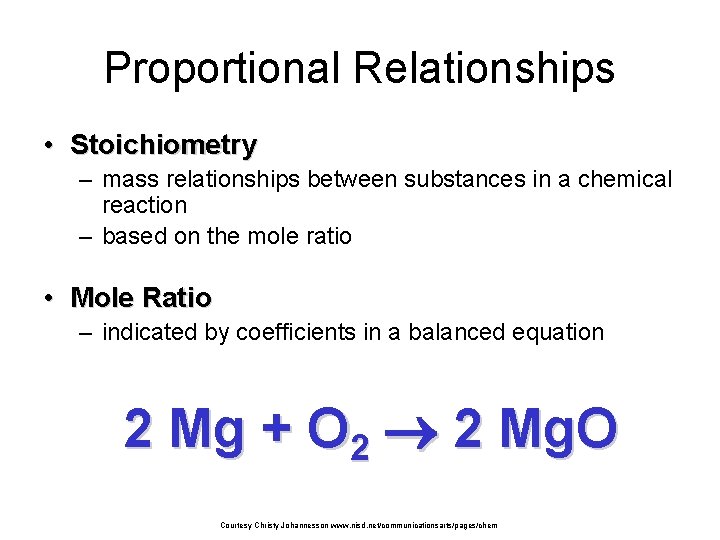
Proportional Relationships • Stoichiometry – mass relationships between substances in a chemical reaction – based on the mole ratio • Mole Ratio – indicated by coefficients in a balanced equation 2 Mg + O 2 2 Mg. O Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

Stoichiometry Steps 1. Write a balanced equation. 2. Identify known & unknown. 3. Line up conversion factors. – – – Mole ratio moles Molarratio mass grams Mole - - moles Molarity moles liters soln Molar volume moles liters gas Core step in all stoichiometry problems!! 4. Check answer. Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

Molar Volume at STP 1 mol of a gas=22. 4 L at STP Standard Temperature & 0°C and 1 atm Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem Pressure

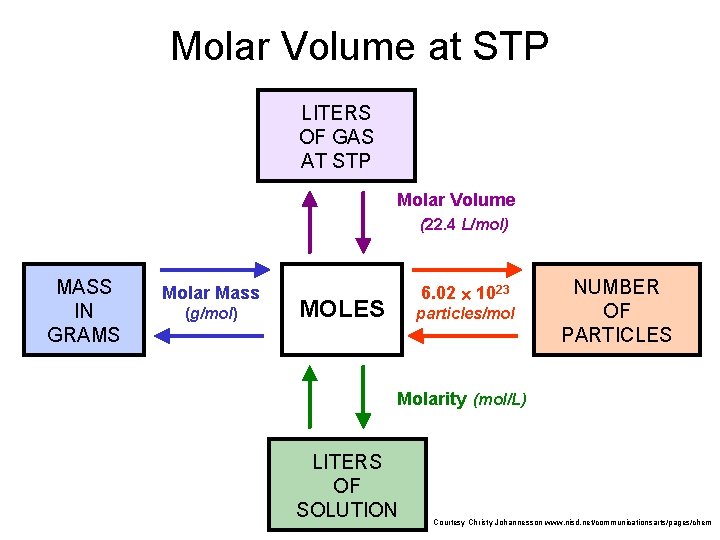
Molar Volume at STP LITERS OF GAS AT STP Molar Volume (22. 4 L/mol) MASS IN GRAMS Molar Mass (g/mol) 6. 02 1023 MOLES particles/mol NUMBER OF PARTICLES Molarity (mol/L) LITERS OF SOLUTION Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

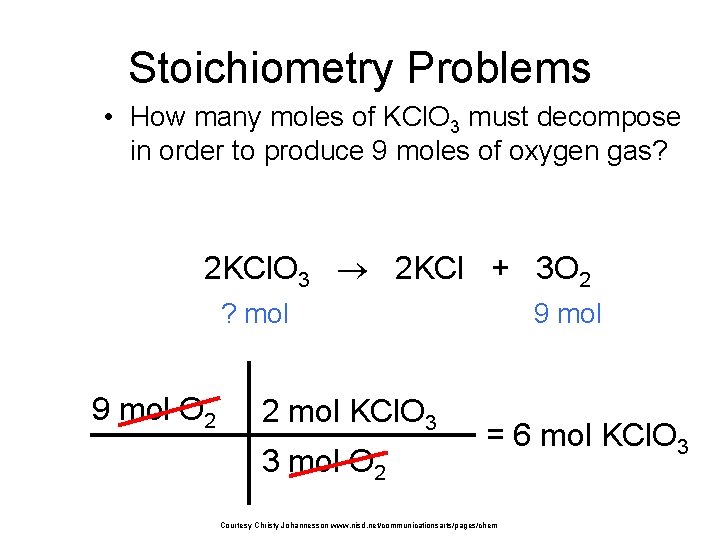
Stoichiometry Problems • How many moles of KCl. O 3 must decompose in order to produce 9 moles of oxygen gas? 2 KCl. O 3 2 KCl + 3 O 2 ? mol 9 mol O 2 2 mol KCl. O 3 3 mol O 2 9 mol = 6 mol KCl. O 3 Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

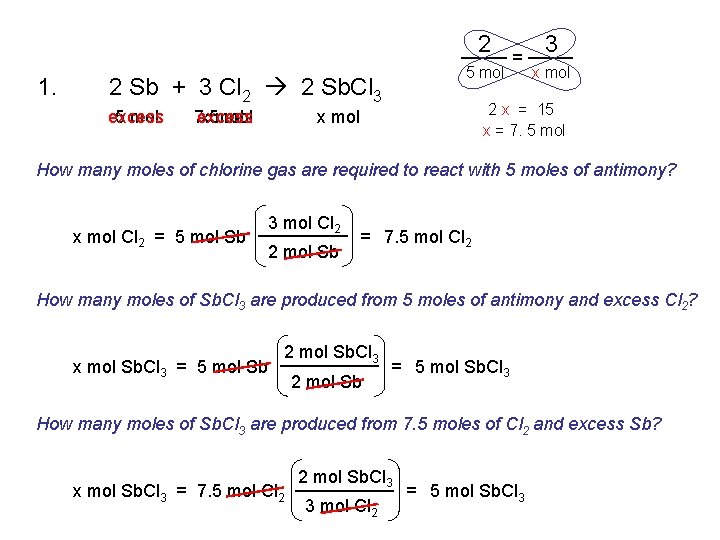
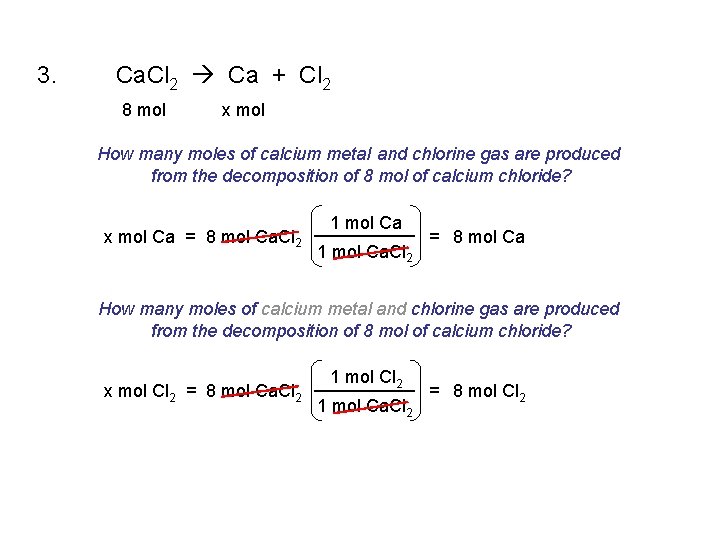
1. 2 Sb + 3 Cl 2 2 Sb. Cl 3 2. 2 Mg + O 2 2 Mg. O 3. Ca. Cl 2 Ca + Cl 2 4. 2 Na. Cl. O 3 2 Na. Cl + 3 O 2 5. Fe + 2 HCl Fe. Cl 2 + H 2 6. Cu. O + H 2 Cu + H 2 O 7. 2 Al + 3 H 2 SO 4 Al 2(SO 4)3 + 3 H 2

2 1. 5 mol 2 Sb + 3 Cl 2 2 Sb. Cl 3 excess 5 mol 7. 5 excess x mol = 3 x mol 2 x = 15 x = 7. 5 mol x mol How many moles of chlorine gas are required to react with 5 moles of antimony? x mol Cl 2 = 5 mol Sb 3 mol Cl 2 2 mol Sb = 7. 5 mol Cl 2 How many moles of Sb. Cl 3 are produced from 5 moles of antimony and excess Cl 2? x mol Sb. Cl 3 = 5 mol Sb 2 mol Sb. Cl 3 2 mol Sb = 5 mol Sb. Cl 3 How many moles of Sb. Cl 3 are produced from 7. 5 moles of Cl 2 and excess Sb? x mol Sb. Cl 3 = 7. 5 mol Cl 2 2 mol Sb. Cl 3 3 mol Cl 2 = 5 mol Sb. Cl 3
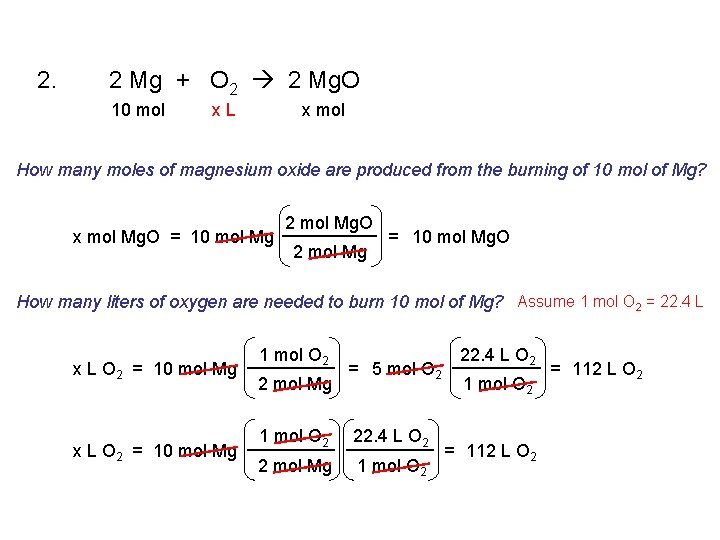
2. 2 Mg + O 2 2 Mg. O 10 mol x. L x mol How many moles of magnesium oxide are produced from the burning of 10 mol of Mg? x mol Mg. O = 10 mol Mg 2 mol Mg. O 2 mol Mg = 10 mol Mg. O How many liters of oxygen are needed to burn 10 mol of Mg? Assume 1 mol O 2 = 22. 4 L x L O 2 = 10 mol Mg 1 mol O 2 2 mol Mg = 5 mol O 2 1 mol O 2 22. 4 L O 2 2 mol Mg 1 mol O 2 22. 4 L O 2 1 mol O 2 = 112 L O 2

3. Ca. Cl 2 Ca + Cl 2 8 mol x mol How many moles of calcium metal and chlorine gas are produced from the decomposition of 8 mol of calcium chloride? x mol Ca = 8 mol Ca. Cl 2 1 mol Ca. Cl 2 = 8 mol Ca How many moles of calcium metal and chlorine gas are produced from the decomposition of 8 mol of calcium chloride? x mol Cl 2 = 8 mol Ca. Cl 2 1 mol Ca. Cl 2 = 8 mol Cl 2

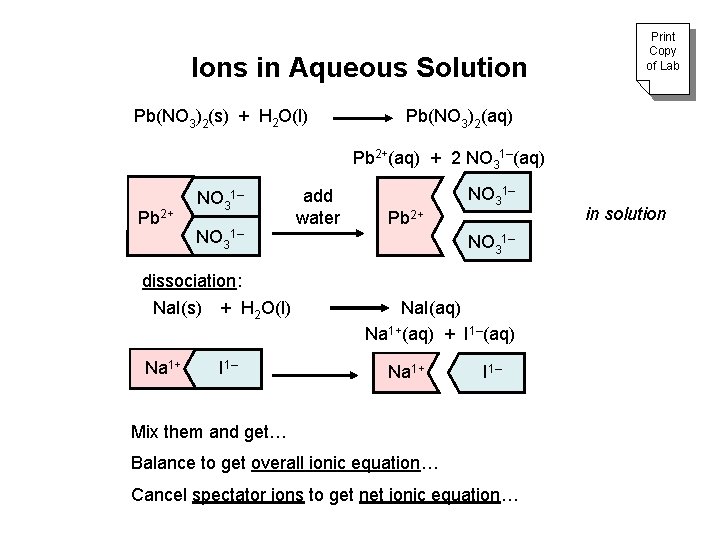
Ions in Aqueous Solution Pb(NO 3)2(s) + H 2 O(l) Print Copy of Lab Pb(NO 3)2(aq) Pb 2+(aq) + 2 NO 31–(aq) Pb 2+ NO 31– dissociation: Na. I(s) + H 2 O(l) Na 1+ I 1– add water Pb 2+ NO 31– Na. I(aq) Na 1+(aq) + I 1–(aq) Na 1+ I 1– Mix them and get… Balance to get overall ionic equation… Cancel spectator ions to get net ionic equation… in solution

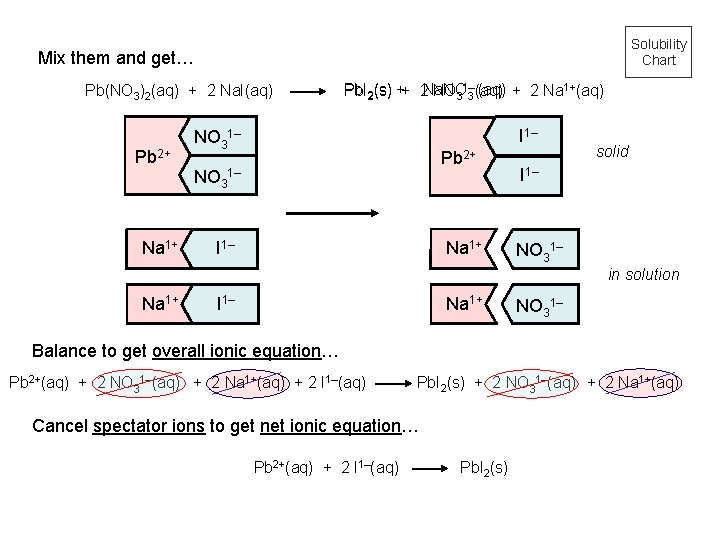
Solubility Chart Mix them and get… Pb(NO 3)2(aq) + 2 Na. I(aq) Pb 2+ Na 1+ Pb. I 2(s) ++ 2 Na. NO (aq) + 2 Na 1+(aq) NO 31– 3 (aq) I 1– NO 31– Pb 2+ NO 31– I 1– Na 1+ solid I 1– NO 31– in solution Na 1+ I 1– Na 1+ NO 31– Balance to get overall ionic equation… Pb 2+(aq) + 2 NO 31–(aq) + 2 Na 1+(aq) + 2 I 1–(aq) Pb. I 2(s) + 2 NO 31–(aq) + 2 Na 1+(aq) Cancel spectator ions to get net ionic equation… Pb 2+(aq) + 2 I 1–(aq) Pb. I 2(s)

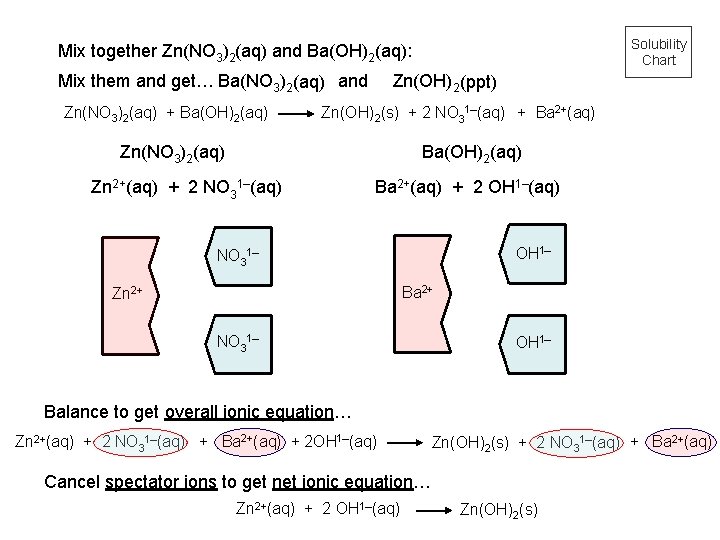
Solubility Chart Mix together Zn(NO 3)2(aq) and Ba(OH)2(aq): Mix them and get… Ba(NO 3)2(aq) and Zn(NO 3)2(aq) + Ba(OH)2(aq) Zn(OH)2 (ppt) Zn(OH)2(s) + 2 NO 31–(aq) + Ba 2+(aq) Zn(NO 3)2(aq) Ba(OH)2(aq) Zn 2+(aq) + 2 NO 31–(aq) Ba 2+(aq) + 2 OH 1–(aq) OH 1– NO 31– Ba 2+ Zn 2+ NO 31– OH 1– Balance to get overall ionic equation… Zn 2+(aq) + 2 NO 31–(aq) + Ba 2+(aq) + 2 OH 1–(aq) Zn(OH)2(s) + 2 NO 31–(aq) + Ba 2+(aq) Cancel spectator ions to get net ionic equation… Zn 2+(aq) + 2 OH 1–(aq) Zn(OH)2(s)
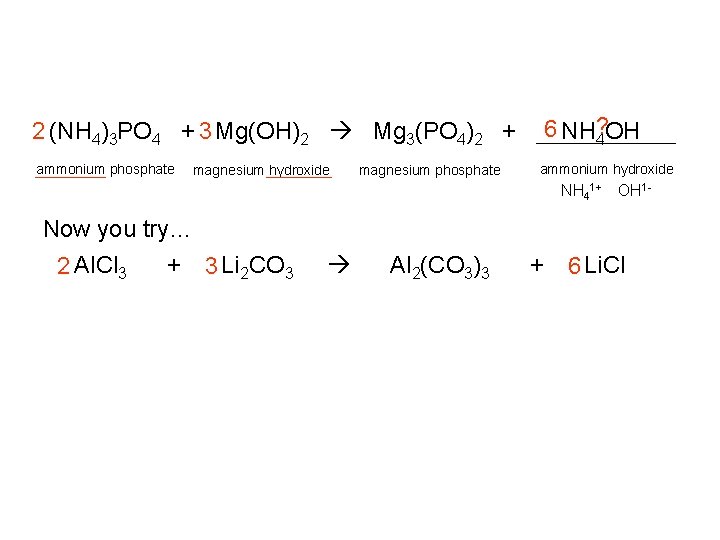
6 NH? 4 OH 2 (NH 4)3 PO 4 + 3 Mg(OH)2 Mg 3(PO 4)2 + ammonium phosphate magnesium hydroxide magnesium phosphate ammonium hydroxide NH 41+ OH 1 - Now you try… 2 Al. Cl 3 + 3 Li 2 CO 3 Al 2(CO 3)3 + 6 Li. Cl

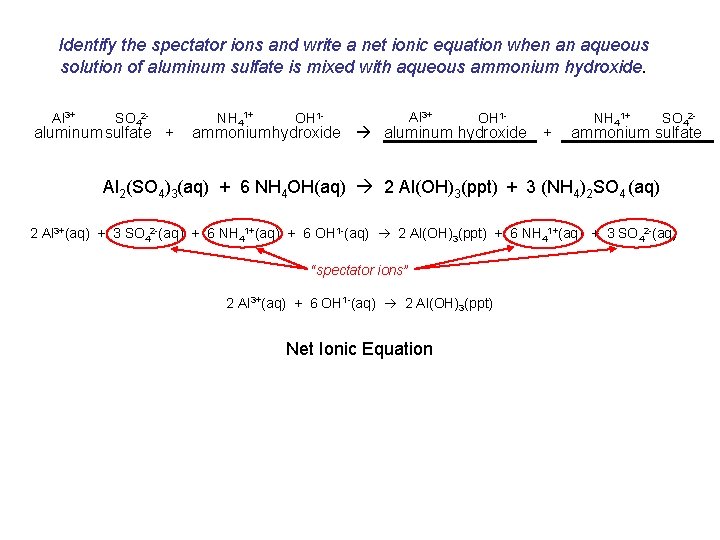
Identify the spectator ions and write a net ionic equation when an aqueous solution of aluminum sulfate is mixed with aqueous ammonium hydroxide. Al 3+ SO 42 - aluminum sulfate + NH 41+ OH 1 - Al 3+ OH 1 - ammoniumhydroxide aluminum hydroxide + NH 41+ SO 42 - ammonium sulfate Al 2(SO 4)3(aq) + 6 NH 4 OH(aq) 2 Al(OH)3(ppt) + 3 (NH 4)2 SO 4 (aq) 2 Al 3+(aq) + 3 SO 42 -(aq) + 6 NH 41+(aq) + 6 OH 1 -(aq) 2 Al(OH)3(ppt) + 6 NH 41+(aq) + 3 SO 42 -(aq) “spectator ions” 2 Al 3+(aq) + 6 OH 1 -(aq) 2 Al(OH)3(ppt) Net Ionic Equation

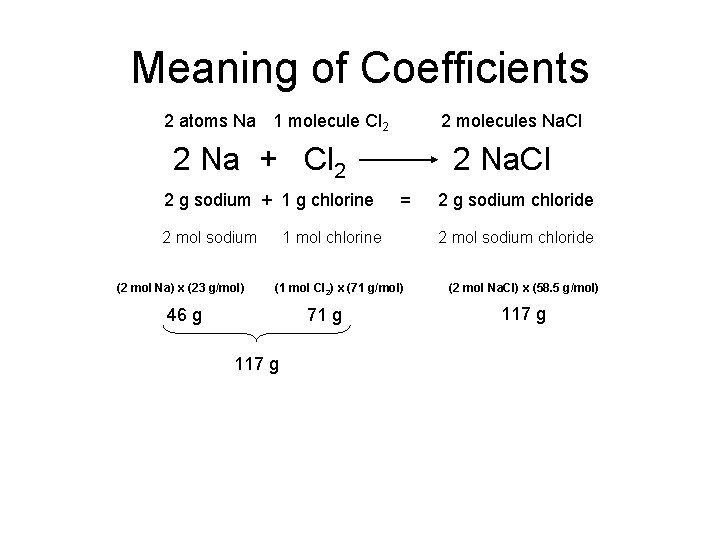
Meaning of Coefficients 2 atoms Na 1 molecule Cl 2 2 molecules Na. Cl 2 Na + Cl 2 2 g sodium + 1 g chlorine 2 mol sodium (2 mol Na) x (23 g/mol) 2 Na. Cl = 1 mol chlorine (1 mol Cl 2) x (71 g/mol) 46 g 71 g 117 g 2 g sodium chloride 2 mol sodium chloride (2 mol Na. Cl) x (58. 5 g/mol) 117 g

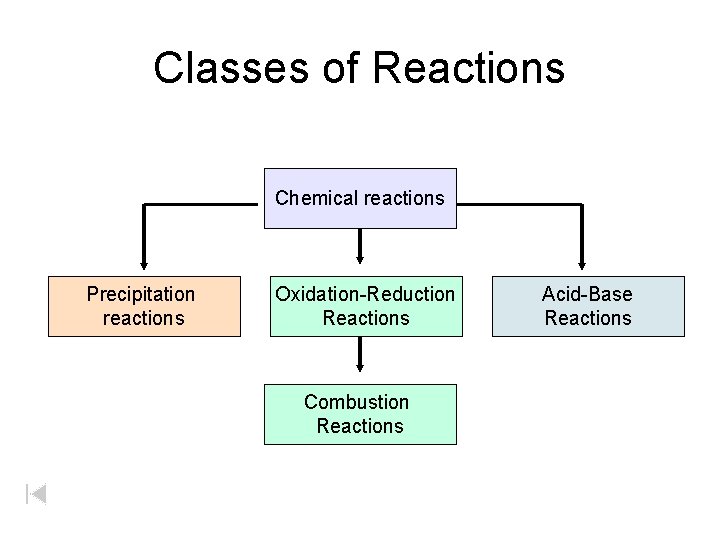
Classes of Reactions Chemical reactions Precipitation reactions Oxidation-Reduction Reactions Combustion Reactions Acid-Base Reactions

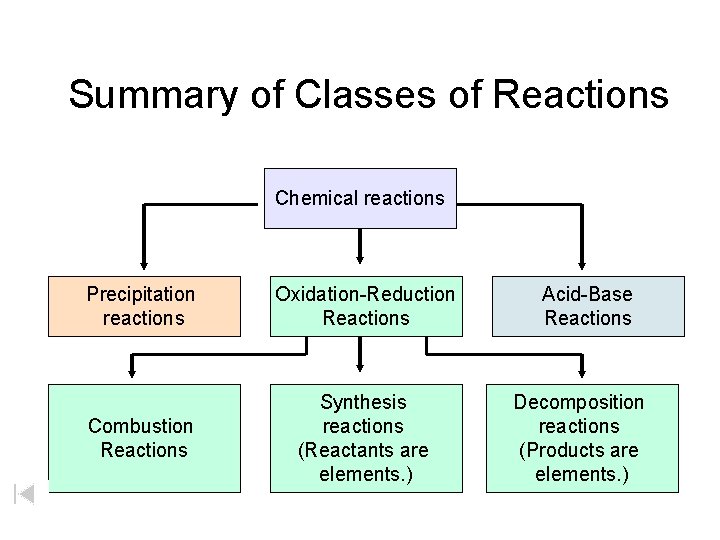
Summary of Classes of Reactions Chemical reactions Precipitation reactions Oxidation-Reduction Reactions Combustion Reactions Synthesis reactions (Reactants are elements. ) Acid-Base Reactions Decomposition reactions (Products are elements. )

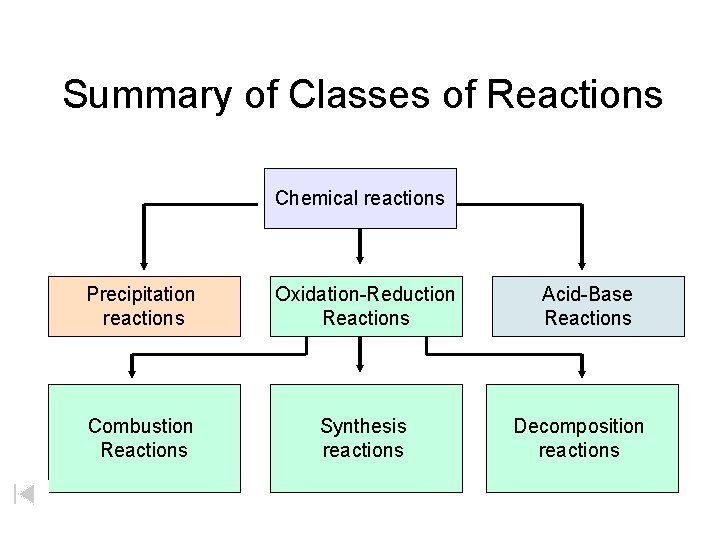
Summary of Classes of Reactions Chemical reactions Precipitation reactions Oxidation-Reduction Reactions Combustion Reactions Synthesis reactions Acid-Base Reactions Decomposition reactions

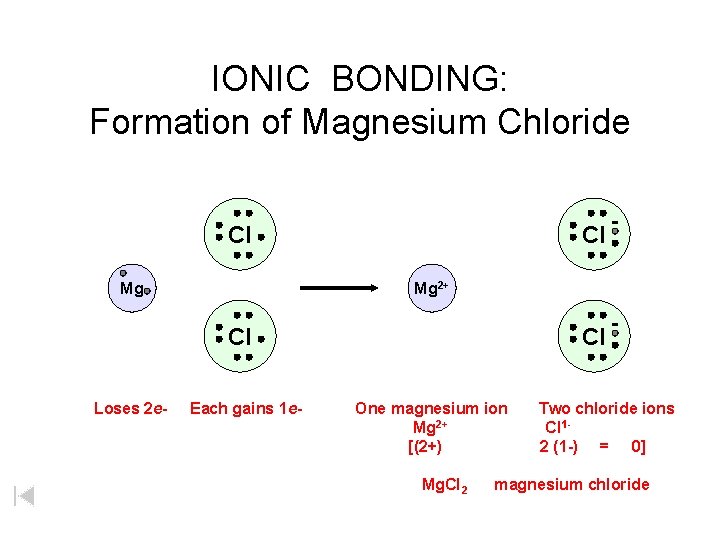
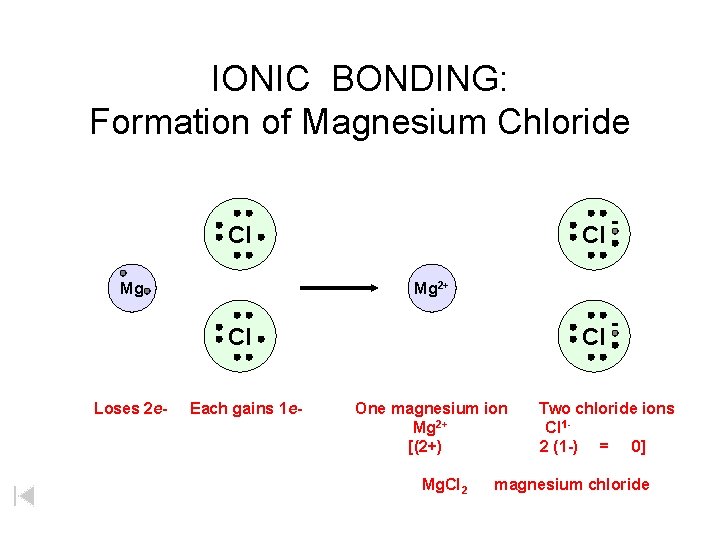
IONIC BONDING: Formation of Magnesium Chloride Cl Mg 2+ Cl Loses 2 e- Each gains 1 e- Cl One magnesium ion Mg 2+ [(2+) Mg. Cl 2 Two chloride ions Cl 12 (1 -) = 0] magnesium chloride

IONIC BONDING: Formation of Magnesium Chloride Cl Mg Mg 2+ Cl Loses 2 e- Each gains 1 e- Cl One magnesium ion Mg 2+ [(2+) Mg. Cl 2 Two chloride ions Cl 12 (1 -) = 0] magnesium chloride

Resources - Chemical Equations and Reactions Worksheet - vocabulary Worksheet – Balancing Chemical Equations Worksheet – Chemical Word Equations Worksheet – Quantitative Relationships in Chem. Eqns. Worksheet – Chemical Equations (paragraph) Worksheet – Real Life Chemistry Worksheet – Balancing Equations (visual) Worksheet Lab – Ions in Solution Textbook - questions Outline (general)