Visual and Safety Performance of the Acry Sof




- Slides: 12

Visual and Safety Performance of the Acry. Sof® Phakic IOL in Global Clinical Trials Prof. Joseph Colin, MD Chef du Service d'Ophtalmologie - CHU Pellegrin Bordeaux, France (Financial Disclosure: Consultant, Alcon) • • • Canada Phase 3 Clinical Investigators T. Demong, MD (Calgary, Alberta) S. Holland, MD (Vancouver, British Columbia) M. Pop, MD (Montreal, Quebec) T. Rabinovitch, MD (Downsview, Ontario) F. Roy, MD (Trois-Rivieres, Quebec) US Phase 2 Clinical Investigators J. Colin, MD ASCRS 2008 §J. D. Horn, MD (Nashville, Tennessee ) §R. Krueger, MD (Cleveland, Ohio) §S. S. Lane, MD (Stillwater, Minnesota) §W. A. Maxwell, MD, Ph. D (Fresno, California) §K. Solomon, MD (Charleston, South Carolina) EU Phase 3 Clinical Investigators • J. Alió, MD (Alicante, Spain) • J. L. Arné, MD (Toulouse, France) • R. Bellucci, MD (Verona, Italy) • B. Cochener MD (Brest, France) • J. Colin, MD (Bordeaux, France) • R. H. Gerl, MD (Ahaus, Germany) • M. Knorz, MD (Mannheim, Germany) • T. Kohnen, MD (Frankfurt, Germany) • A. Marinho, MD/F. Vaz, MD (Porto, Portugal)

Clinical Study Design l l l Clinical Objective: Investigate safety & effectiveness Study design: Non-randomized, open label, single arm, multicenter clinical investigations Pooled analysis of global clinical studies l 360 subjects included in total analysis l l l 3 to 5 year follow-up duration Results reported for 2 year follow-up visit (n=204) Not approved for general use. J. Colin, MD ASCRS 2008 European Phase 3 Clinical Study: 190 subjects Canadian Phase 3 Clinical Study: 120 subjects US Phase 2 Clinical Study: 50 subjects L-Series Acry. Sof® PIOL l Optic 6. 0 mm l Overall length 12. 5 mm to 14. 0 mm l Diopter Range -6. 0 D to -16. 5 D l l l Anterior Chamber Angle-Supported Single-Piece IOL design Soft Acrylic (Acry. Sof® IOL material) Monarch® II IOL Delivery System Small incision size

Study Criteria l Main Inclusion Criteria l Adults >18 years of age, with cap of 49 years of age for US and Canadian study l Stable, moderate to high myopia (refraction within ± 0. 5 D at least 12 months prior to surgery) l Able & willing to sign informed consent l Main Exclusion Criteria l l l J. Colin, MD ASCRS 2008 Previous ocular surgery Glaucoma or family history of glaucoma Mesopic pupil size >7. 0 mm Astigmatism >2. 0 D Anterior chamber depth <3. 2 mm Non-qualifying endothelial cell density per age criteria

Postoperative Evaluation l l l l J. Colin, MD ASCRS 2008 Endothelial Cell Density (ECD) & Morphology Adverse Events (AE) Maintenance of Best Spectacle-Corrected Visual Acuity (BSCVA) Uncorrected Distance Visual Acuity (UCVA) Best Spectacle-Corrected Distance Visual Acuity (BSCVA) Manifest Refraction Spherical Equivalent (MRSE) Predictability of Refraction

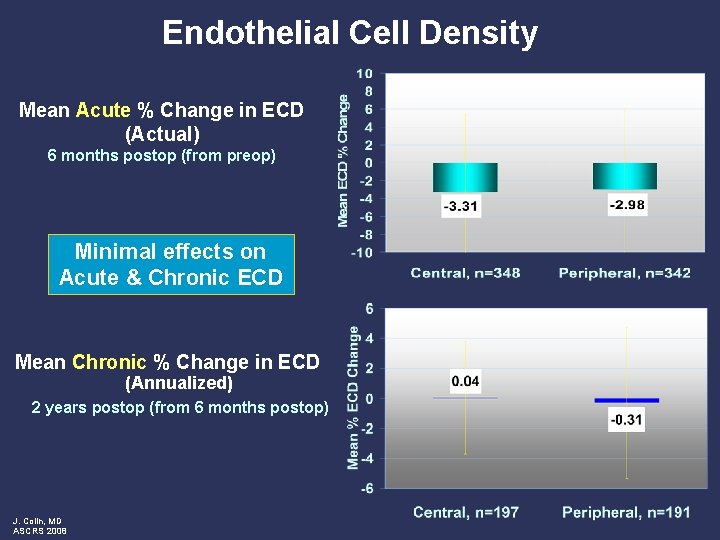
Endothelial Cell Density Mean Acute % Change in ECD (Actual) 6 months postop (from preop) Minimal effects on Acute & Chronic ECD Mean Chronic % Change in ECD (Annualized) 2 years postop (from 6 months postop) J. Colin, MD ASCRS 2008

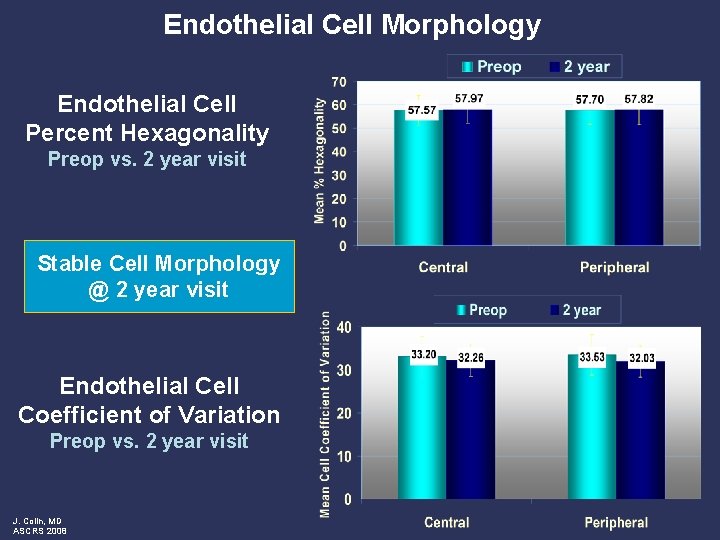
Endothelial Cell Morphology Endothelial Cell Percent Hexagonality Preop vs. 2 year visit Stable Cell Morphology @ 2 year visit Endothelial Cell Coefficient of Variation Preop vs. 2 year visit J. Colin, MD ASCRS 2008

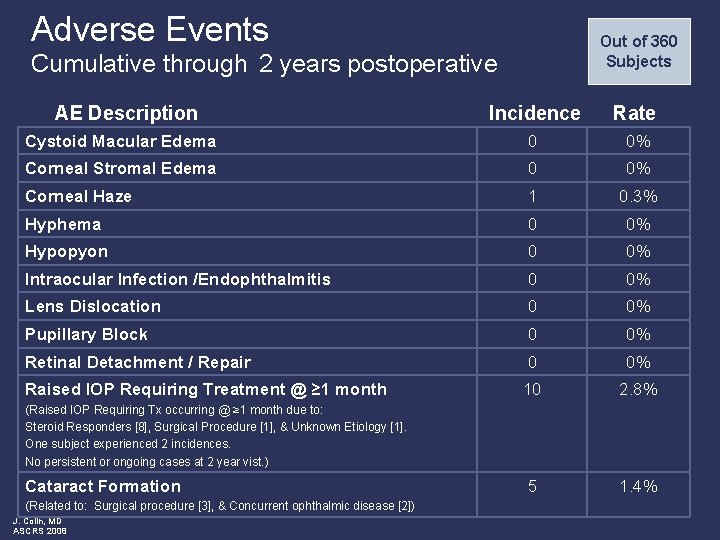
Adverse Events Out of 360 Subjects Cumulative through 2 years postoperative AE Description Incidence Rate Cystoid Macular Edema 0 0% Corneal Stromal Edema 0 0% Corneal Haze 1 0. 3% Hyphema 0 0% Hypopyon 0 0% Intraocular Infection /Endophthalmitis 0 0% Lens Dislocation 0 0% Pupillary Block 0 0% Retinal Detachment / Repair 0 0% Raised IOP Requiring Treatment @ ≥ 1 month 10 2. 8% 5 1. 4% (Raised IOP Requiring Tx occurring @ ≥ 1 month due to: Steroid Responders [8], Surgical Procedure [1], & Unknown Etiology [1]. One subject experienced 2 incidences. No persistent or ongoing cases at 2 year vist. ) Cataract Formation (Related to: Surgical procedure [3], & Concurrent ophthalmic disease [2]) J. Colin, MD ASCRS 2008

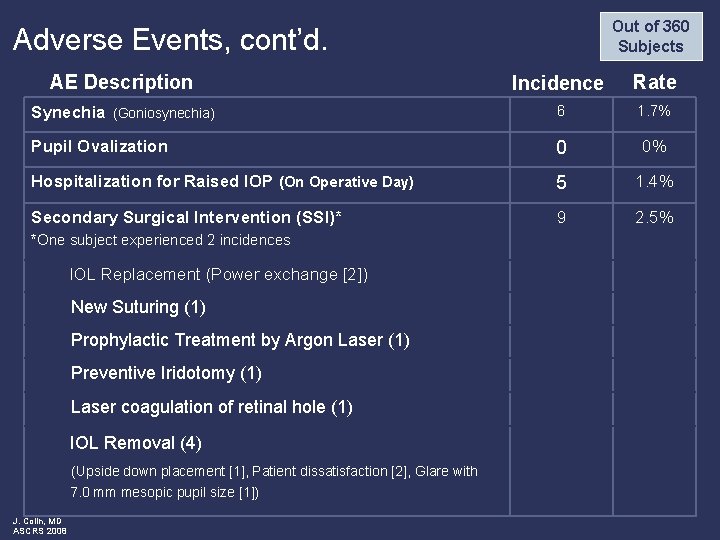
Out of 360 Subjects Adverse Events, cont’d. AE Description Incidence Rate Synechia (Goniosynechia) 6 1. 7% Pupil Ovalization 0 0% Hospitalization for Raised IOP (On Operative Day) 5 1. 4% Secondary Surgical Intervention (SSI)* 9 2. 5% *One subject experienced 2 incidences IOL Replacement (Power exchange [2]) New Suturing (1) Prophylactic Treatment by Argon Laser (1) Preventive Iridotomy (1) Laser coagulation of retinal hole (1) IOL Removal (4) (Upside down placement [1], Patient dissatisfaction [2], Glare with 7. 0 mm mesopic pupil size [1]) J. Colin, MD ASCRS 2008

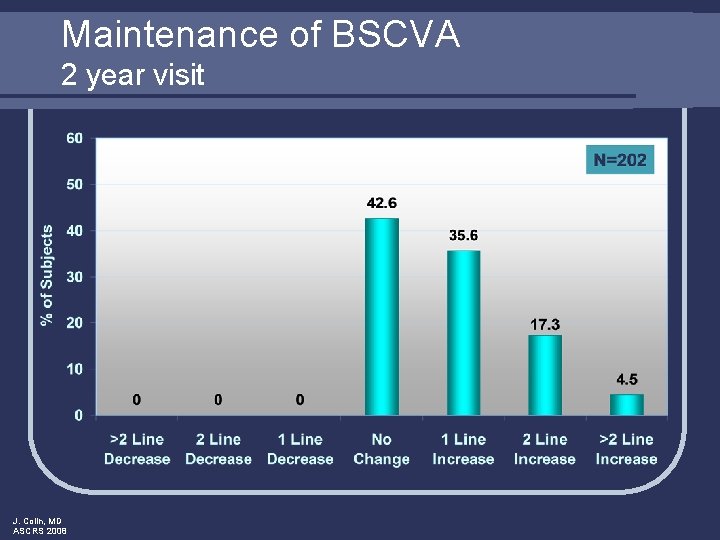
Maintenance of BSCVA 2 year visit J. Colin, MD ASCRS 2008

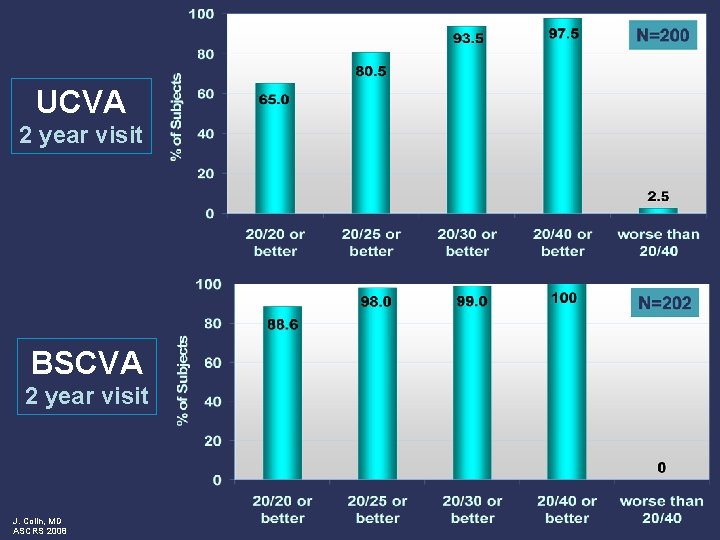
UCVA 2 year visit BSCVA 2 year visit J. Colin, MD ASCRS 2008

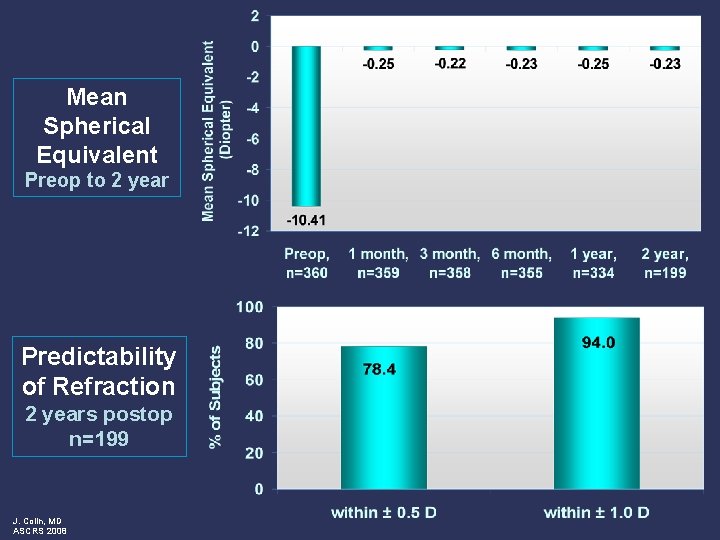
Mean Spherical Equivalent Preop to 2 year Predictability of Refraction 2 years postop n=199 J. Colin, MD ASCRS 2008

Conclusions: 2 year follow-up visit l Safety Outcomes l l l Effectiveness Outcomes l l J. Colin, MD ASCRS 2008 Minimal effects on ECD at 2 years postop BSCVA maintained No chronic inflammation or persistently raised IOP Low rate of adverse events observed Excellent visual acuity High rate of predictability Refraction stable over time Clinical investigations ongoing