Visit https checkin ics uci edu Log in



![Rank in order of increasing radius: • Carbon, Nitrogen and oxygen C: [He]2 s Rank in order of increasing radius: • Carbon, Nitrogen and oxygen C: [He]2 s](https://slidetodoc.com/presentation_image/d91e539440cb2a0c47d93faded348176/image-6.jpg)




- Slides: 16


• Visit: https: //checkin. ics. uci. edu/ • Log in and select Chem 1 A. • When prompted, type the word of the day: ionic • Ensure when asked if you will share your location you select “allow”. • Visit: https: //learningcatalytics. com/ • Sign in Mastering. Chemistry account name When prompted, type session ID: 89555625 Plan ordering for the day: Finish up periodic trends “exceptions” Review a couple homework problems together Alone “quiz” questions for day. More homework questions Ionic and covalent bonding powerpoint. Remember back 5 rows of even side are no seating zones

Rank in order of effective nuclear charge: • Carbon, Nitrogen and oxygen Increasing: effective nuclear charge C, N, O Visit: https: //checkin. ics. uci. edu/ • Word of the day: ionic Ensure when asked if you will share your location you select “allow”. Learning. Catalytics: session 89555625

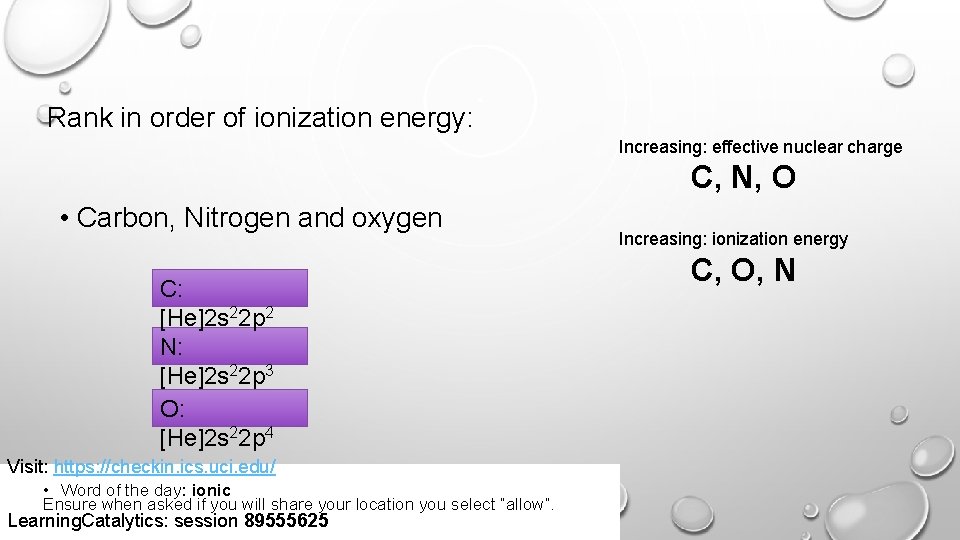
Rank in order of ionization energy: Increasing: effective nuclear charge C, N, O • Carbon, Nitrogen and oxygen C: [He]2 s 22 p 2 N: [He]2 s 22 p 3 O: [He]2 s 22 p 4 Visit: https: //checkin. ics. uci. edu/ • Word of the day: ionic Ensure when asked if you will share your location you select “allow”. Learning. Catalytics: session 89555625 Increasing: ionization energy C, O, N

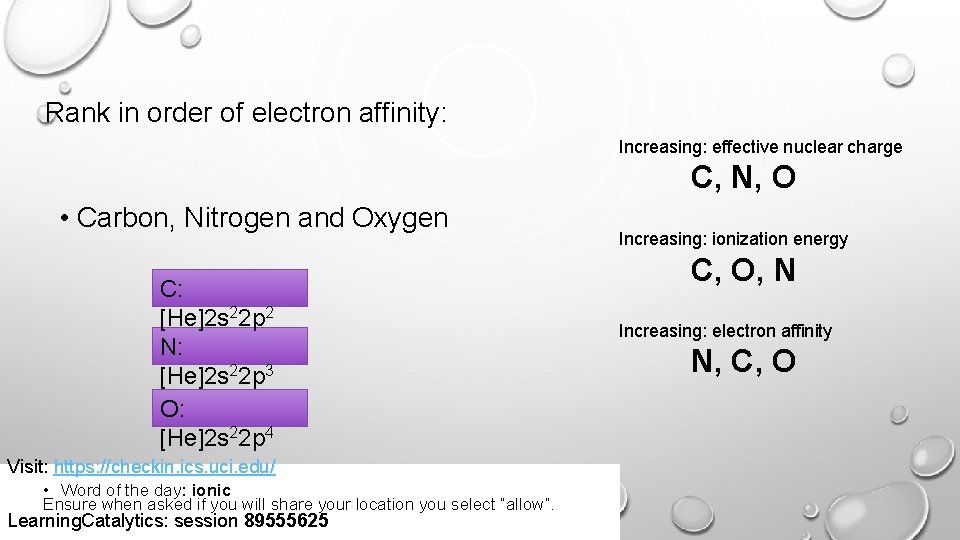
Rank in order of electron affinity: Increasing: effective nuclear charge C, N, O • Carbon, Nitrogen and Oxygen C: [He]2 s 22 p 2 N: [He]2 s 22 p 3 O: [He]2 s 22 p 4 Visit: https: //checkin. ics. uci. edu/ • Word of the day: ionic Ensure when asked if you will share your location you select “allow”. Learning. Catalytics: session 89555625 Increasing: ionization energy C, O, N Increasing: electron affinity N, C, O
![Rank in order of increasing radius Carbon Nitrogen and oxygen C He2 s Rank in order of increasing radius: • Carbon, Nitrogen and oxygen C: [He]2 s](https://slidetodoc.com/presentation_image/d91e539440cb2a0c47d93faded348176/image-6.jpg)
Rank in order of increasing radius: • Carbon, Nitrogen and oxygen C: [He]2 s 22 p 2 N: [He]2 s 22 p 3 O: [He]2 s 22 p 4 Visit: https: //checkin. ics. uci. edu/ • Word of the day: ionic Ensure when asked if you will share your location you select “allow”. Learning. Catalytics: session 89555625 Increasing: effective nuclear charge C, N, O Increasing: ionization energy C, O, N Increasing: electron affinity N, C, O Increasing radius O, N, C

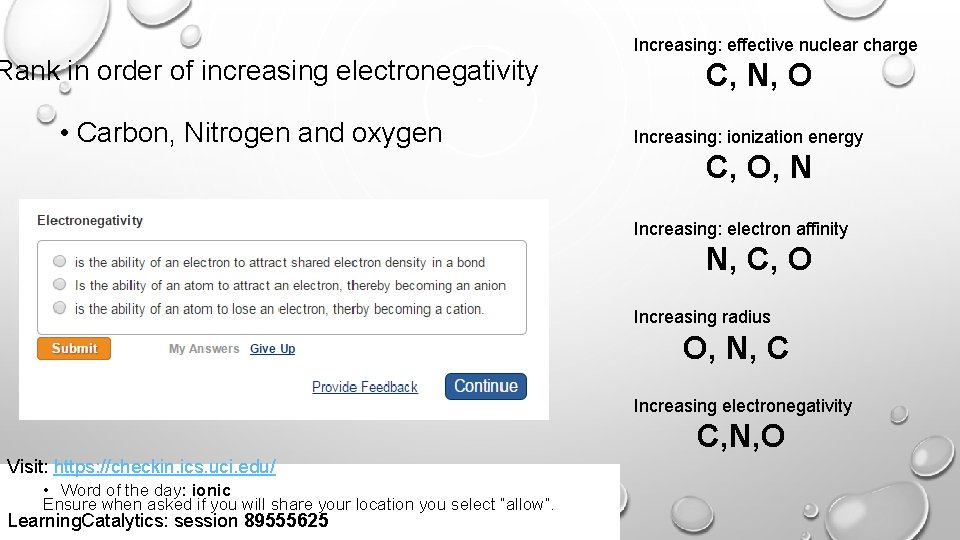
Increasing: effective nuclear charge Rank in order of increasing electronegativity • Carbon, Nitrogen and oxygen C, N, O Increasing: ionization energy C, O, N Increasing: electron affinity N, C, O Increasing radius O, N, C Increasing electronegativity C, N, O Visit: https: //checkin. ics. uci. edu/ • Word of the day: ionic Ensure when asked if you will share your location you select “allow”. Learning. Catalytics: session 89555625

Ionic and Covalent Bonding Including Naming Note: We likely won’t make it to covalent nomenclature, this is the one students find FAR easier than ionic. Please refer to the videos and naming hand out for help with this, and as always office hours, discussions, and facebook for extra help. If you don’t remember it from high school/1 P. Visit: https: //checkin. ics. uci. edu/ • Word of the day: ionic Ensure when asked if you will share your location you select “allow”. Learning. Catalytics: session 89555625

HOMEWORK REVIEW K +, I - one electron traded …. Extending, for Mg. O, how many electrons are traded? Mg 2+, O 2 - two electrons traded

“quiz” question (remember, work alone but use whatever you want to help you) Visit: https: //checkin. ics. uci. edu/ • Word of the day: ionic Ensure when asked if you will share your location you select “allow”. Learning. Catalytics: session 89555625

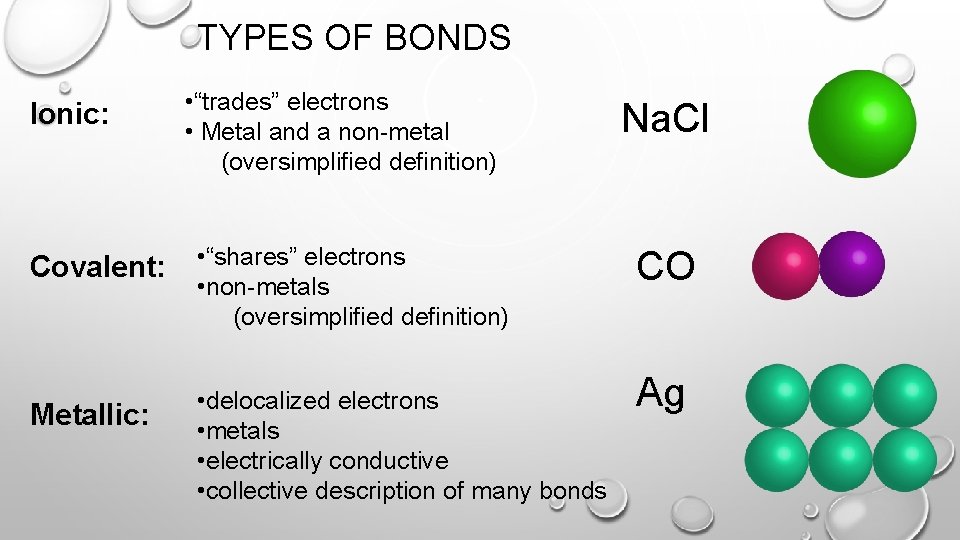
TYPES OF BONDS Ionic: • “trades” electrons • Metal and a non-metal (oversimplified definition) Covalent: • “shares” electrons • non-metals (oversimplified definition) Metallic: • delocalized electrons • metals • electrically conductive • collective description of many bonds Na. Cl CO Ag

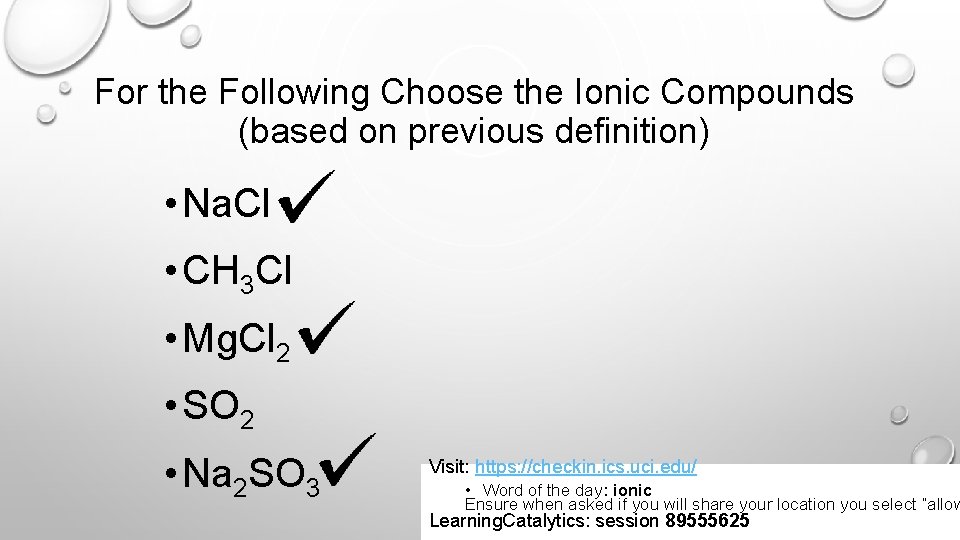
For the Following Choose the Ionic Compounds (based on previous definition) • Na. Cl • CH 3 Cl • Mg. Cl 2 • SO 2 • Na 2 SO 3 Visit: https: //checkin. ics. uci. edu/ • Word of the day: ionic Ensure when asked if you will share your location you select “allow Learning. Catalytics: session 89555625

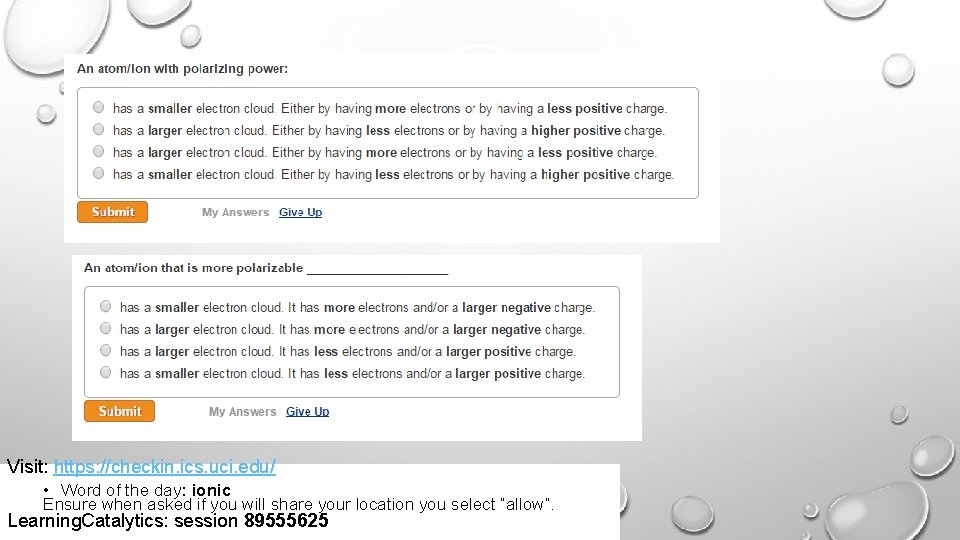
Visit: https: //checkin. ics. uci. edu/ • Word of the day: ionic Ensure when asked if you will share your location you select “allow”. Learning. Catalytics: session 89555625

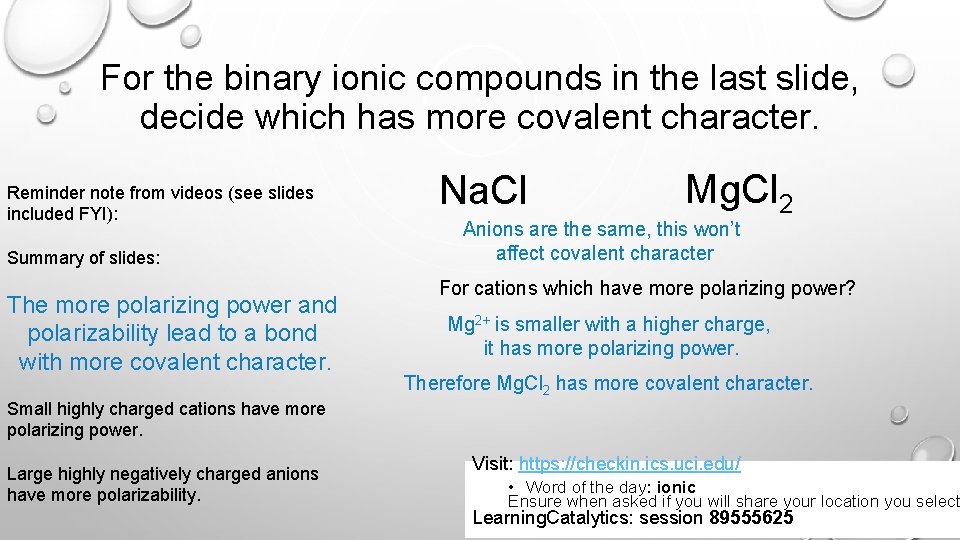
For the binary ionic compounds in the last slide, decide which has more covalent character. Reminder note from videos (see slides included FYI): Summary of slides: The more polarizing power and polarizability lead to a bond with more covalent character. Na. Cl Mg. Cl 2 Anions are the same, this won’t affect covalent character For cations which have more polarizing power? Mg 2+ is smaller with a higher charge, it has more polarizing power. Therefore Mg. Cl 2 has more covalent character. Small highly charged cations have more polarizing power. Large highly negatively charged anions have more polarizability. Visit: https: //checkin. ics. uci. edu/ • Word of the day: ionic Ensure when asked if you will share your location you select Learning. Catalytics: session 89555625

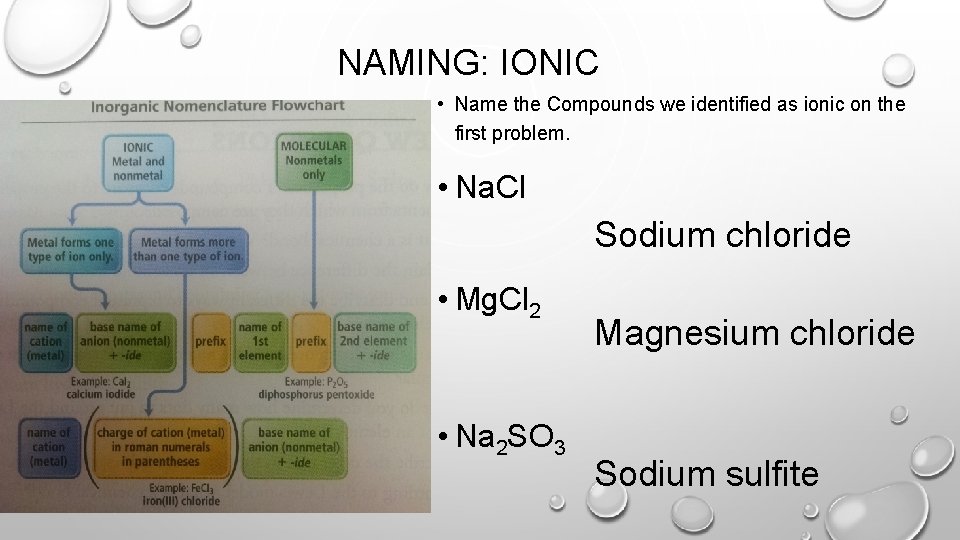
NAMING: IONIC • Name the Compounds we identified as ionic on the first problem. • Na. Cl Sodium chloride • Mg. Cl 2 • Na 2 SO 3 Magnesium chloride Sodium sulfite

NAMING: COVALENT • Name the Compounds below. • SO 2 Sulfur dioxide • NO Nitrogen monoxide • PCl 5 Phosphorous pentachloride