Viruses Associated with Gastroenteritis Ghazi Jamjoom VIRAL AGENTS

































![Diagnosis- Human Caliciviruses • Specimen- stool , vomitus, environmental swabs, [not yet on foods] Diagnosis- Human Caliciviruses • Specimen- stool , vomitus, environmental swabs, [not yet on foods]](https://slidetodoc.com/presentation_image/1d789a1d1d2908b904d41582e92b0710/image-60.jpg)









































- Slides: 120

Viruses Associated with Gastroenteritis Ghazi Jamjoom

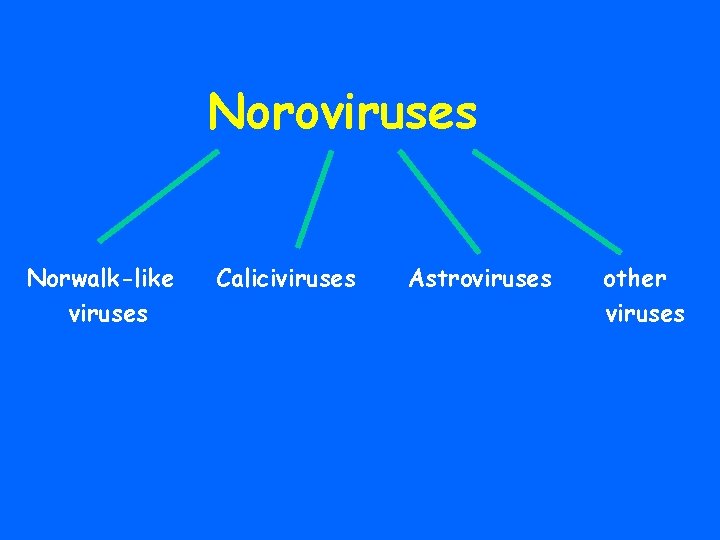
VIRAL AGENTS CAUSING GASTROENTERITIS Major Viruses 1. Rotavirus 2. Enteric adenoviruses 3. Noroviruses : 4. a. Norwalk-like viruses 5. b. Calicivirus

Noroviruses Norwalk-like viruses Caliciviruses Astroviruses other viruses

Viruses associated with gastroenteritis (cont) : Other viruses (minor): • Coronaviruses • Parvoviruses • Pestiviruses • Toroviruses

ROTAVIRUS Family Reoviridae Genus Rotavirus

ROTAVIRUS • First isolated in 1973 from children with diarrhea • EM identification from duodenal biopsies • Human and animal strains

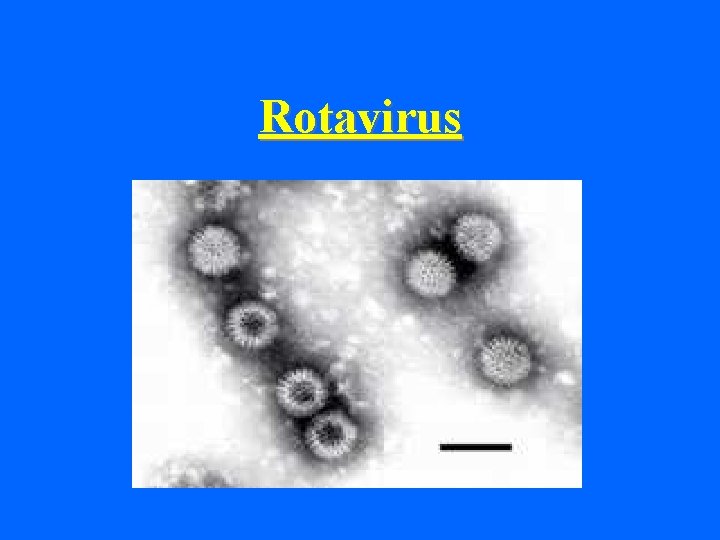
Rotavirus

Rotavirus- EM Structure

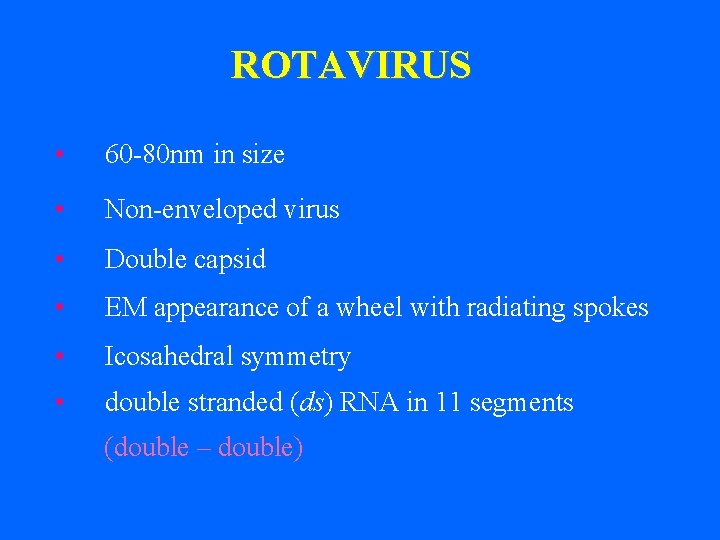
ROTAVIRUS • 60 -80 nm in size • Non-enveloped virus • Double capsid • EM appearance of a wheel with radiating spokes • Icosahedral symmetry • double stranded (ds) RNA in 11 segments (double – double)

ROTAVIRUS- 3 D STRUCTURE

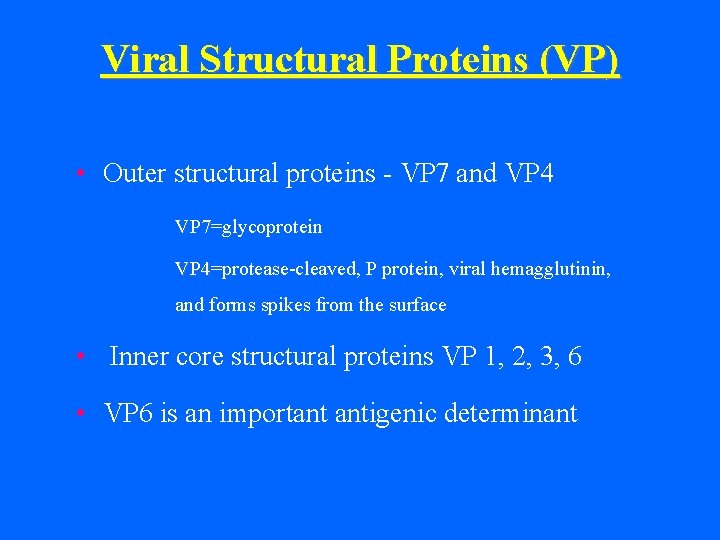
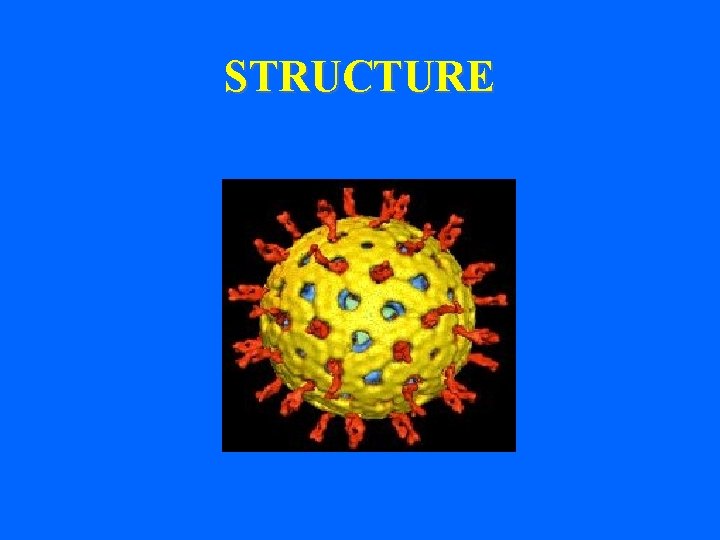
Viral Structural Proteins (VP) • Outer structural proteins - VP 7 and VP 4 VP 7=glycoprotein VP 7 VP 4=protease-cleaved, P protein, viral hemagglutinin, VP 4 and forms spikes from the surface • Inner core structural proteins VP 1, 2, 3, 6 • VP 6 is an important antigenic determinant

• Genome is composed of 11 segments of double-stranded RNA, six structural coding for proteins five nonstructural • Seven serological groups have been identified (A-G), three of which (groups A, B, and C) infect humans.

STRUCTURE

Gene coding assignment

Classification • Groups, subgoups, serotypes based on viral capsid proteins • 7 Groups (A through G) • Group A is the most common and has 2 subgroups • 10 human serotypes based on G protein (VP 7) • 8 P protein serotypes

Classification (contd. ) • Electropherotypes mobility of RNA segments by PAGE Used in epidemiologic studies


Rotavirus - Properties • Virus is stable in the environment • Relatively resistant to handwashing agents • Susceptible to disinfection with 95% ethanol, ‘Lysol’, formalin

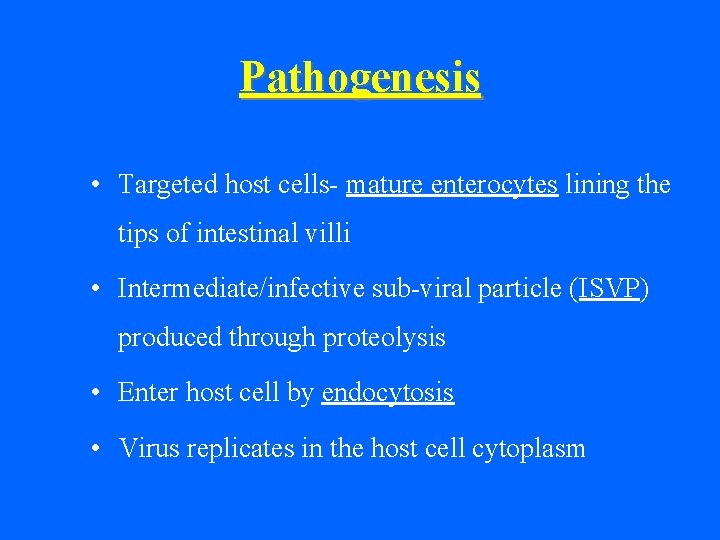
Pathogenesis • Targeted host cells- mature enterocytes lining the tips of intestinal villi • Intermediate/infective sub-viral particle (ISVP) produced through proteolysis • Enter host cell by endocytosis • Virus replicates in the host cell cytoplasm

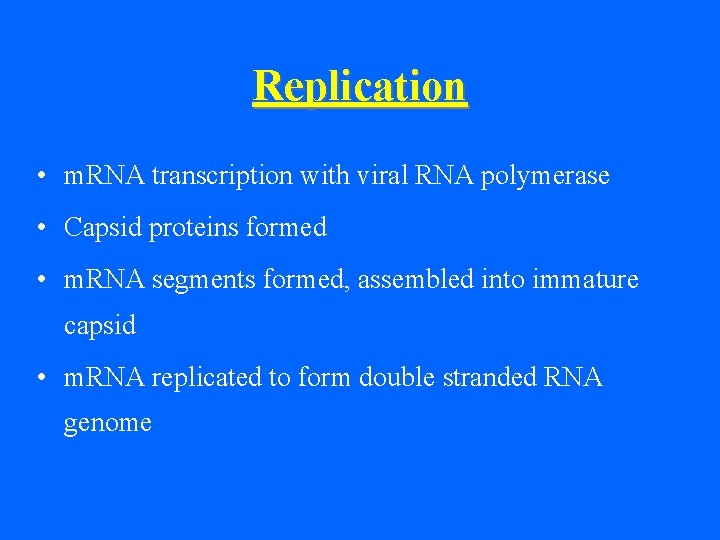
Replication • m. RNA transcription with viral RNA polymerase • Capsid proteins formed • m. RNA segments formed, assembled into immature capsid • m. RNA replicated to form double stranded RNA genome

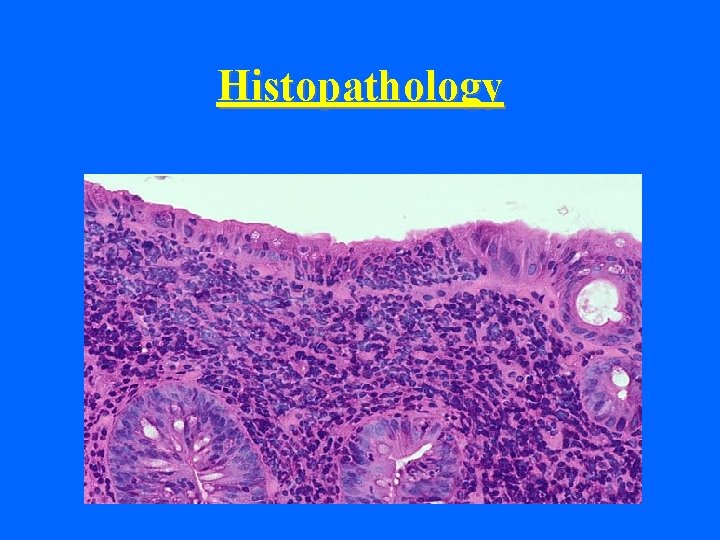
Histopathology • Mature enterocytes lining the tips of intestinal villi are affected • Villous atrophy and blunting • Death of the mature enterocytes

Histopathology

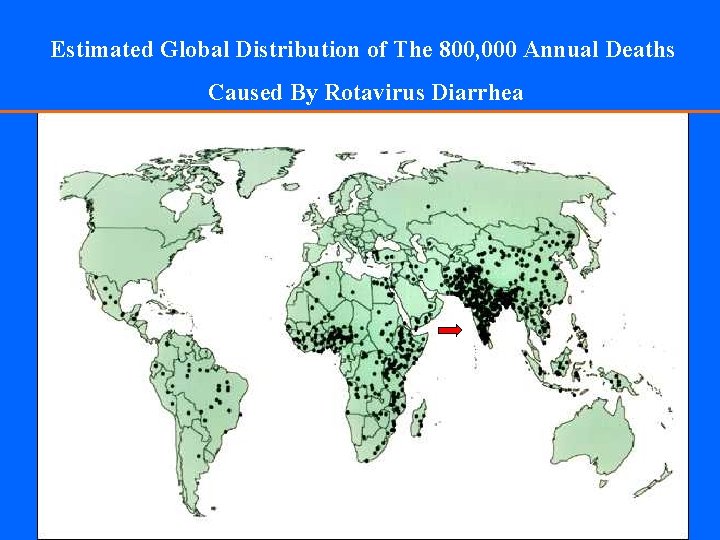
Epidemiology - Worldwide • Millions are affected • 600, 000 -850, 000 deaths/year • A major cause of diarrhea-associated hospitalizations • Seroprevalence studies show that antibody is present in most by age 3 y.

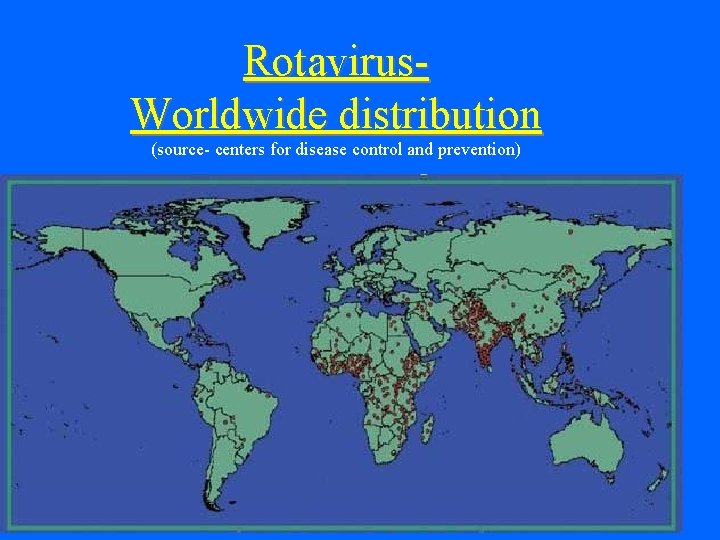
Rotavirus. Worldwide distribution (source- centers for disease control and prevention)

Estimated Global Distribution of The 800, 000 Annual Deaths Caused By Rotavirus Diarrhea

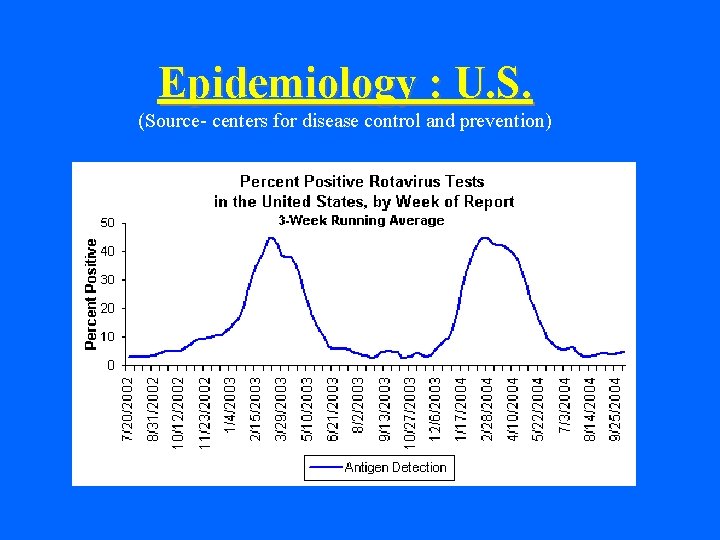
Epidemiology : U. S. • No. of children under 5 y. affected ~ 2. 7 million • Physician visits per year ~ 500, 000 • Hospitalizations per year ~ 50, 000 • Deaths per year ~ 20 - 40 • % cases w/ dehydration ~ 1 -2. 5%

Epidemiology • Age- 4 mo - 2 years Protection of younger infants through transplacental antibody transfer • Asymptomatic infections are common, especially in adults • Nosocomial infections • Outbreaks

Epidemiology (contd. ) • Seasonality Winter months (Nov. through May in US) Gradual spread W to E Year-round in the tropics • Incubation period - thought to be <4 days

Epidemiology : U. S. (Source- centers for disease control and prevention)

Epidemiology (transmission) • Mainly person to person via fecal-oral route • Fomites • Food and water-borne spread is possible • Spread via respiratory route is speculated

Epidemiology (spread) • Contagious from before onset of diarrhea to a few days after end of diarrhea • Large amounts of viral particles are shed in diarrheal stools • Infective dose is only 10 -100 pfu

EPIDEMIOLOGY Differences in Groups • Group A infections are most common • Group B has been associated with outbreaks in adults in China • Group C is responsible for sporadic cases of diarrhea in infants around the world

Clinical Features • Incubation period - thought to be <4 days • Fever- can be high grade (>102 F in 30%) • Vomiting, nausea precede diarrhea • Diarrhea - usually watery (no blood or leukocytes) - lasts 3 -9 days - longer in malnourished and immune deficient indiv. - NEC and hemorrhagic GE seen in neonates

Mechanism of diarrhea • Watery diarrhea due to net secretion of intestinal fluid • Activation of the enteric nervous system possible role of enterotoxin

Clinical Features (contd. ) • Dehydration is the main contributor to mortality. • Secondary malabsorption of lactose and fat, and chronic diarrhea are possible

• Recovery is usually complete. • However, severe diarrhea without fluid and electrolyte replacement may result in dehydration and death.

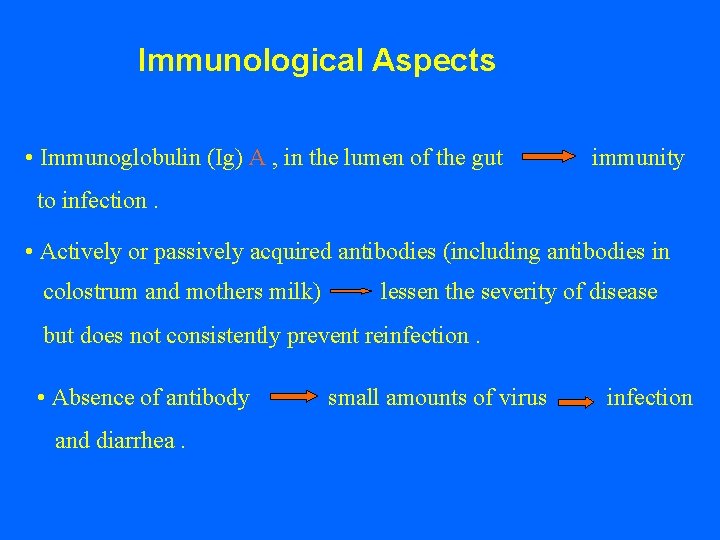
Immunological Aspects • Immunoglobulin (Ig) A , in the lumen of the gut immunity to infection. • Actively or passively acquired antibodies (including antibodies in colostrum and mothers milk) lessen the severity of disease but does not consistently prevent reinfection. • Absence of antibody small amounts of virus infection and diarrhea.

Diagnosis • Antigen detection in stool by ELISA, Latex Agglutination (for Group A rotavirus) • EM- non-Group A viruses also • Culture- Group A rotaviruses can be cultured in monkey kidney cells • Serology for epidemiologic studies

Treatment and Prevention • Treatment- Supportive - oral, IV rehydration • Prevention Handwashing and disinfection of surfaces

Vaccine • Live tetravalent rhesus-human reassortant vaccine (Rotashield) • Licensed for use in August 1998 • Removed from the market in October 1999 due to risk of intussusception • Cases were seen 3 -20 days after vaccination • Approx. 15 cases/1. 5 million doses • New vaccine from bovine rotavirus under trial

Production of rhesus rotavirus (RRV), human rotavirus (HRV) x rhesus rotavirus (RRV)reassortant quadrivalent vaccine wit VP 7 serotype 1, 2, 3, and 4 specificity

GASTROENTERITIS DUE TO ENTERIC ADENOVIRUS

GASTROENTERITIS DUE TO ADENOVIRUS • Types 40, 41 • Belong to serogroup F • Some cases due to types 31, 3, 7

Diarrhea due to Enteric Adenovirus • Age <4 years • Year round • Spread via fecal-oral route

Clinical features of Enteric Adenovirus gastroenteritis • Incubation period 3 -10 days • Diarrhea lasts for 10 -14 days • Can also cause intussusception, mesenteric adenitis, appendicitis

Diagnosis- Enteric adenoviruses • Isolation requires special media-Graham 293 • ELISA for rapid detection is available

HUMAN CALICIVIRUSES

HUMAN CALICIVIRUSES (Hu. CV) • Belong to Family Caliciviridae • Non-enveloped RNA viruses with ss RNA • 27 -35 nm in size • Contain a single capsid protein

HUMAN CALICIVIRUSES • Genomic analysis divides it into 4 groups • Human caliciviruses belong to 2 genera

CLASSIFICATION OF Hu. CV NLV (Norovirus) Norwalk virus Hawaii virus Snow Mountain virus Montgomery county virus Taunton (England) SLV (Sapovirus) Sapporo virus Manchester virus Houston/86 London/92

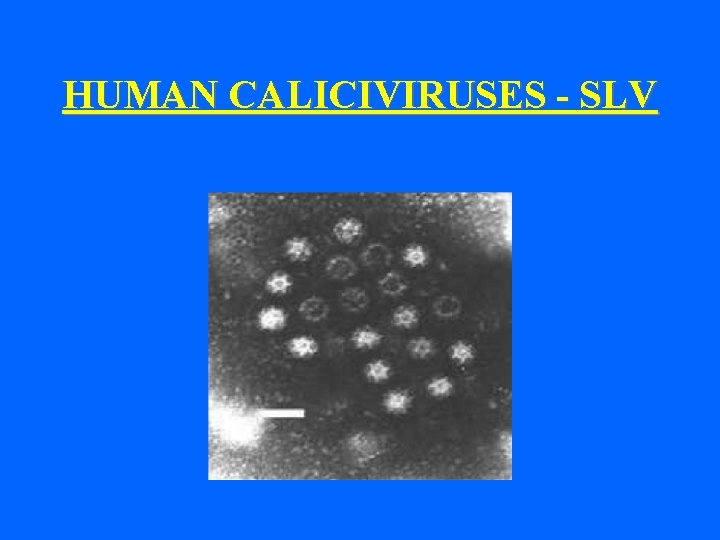
Morphology of Hu. CV- typical • Typical morphology • 32 cup-like depressions • EM appearance of “Star of David” E. g. - Sapporo-like viruses

HUMAN CALICIVIRUSES - SLV

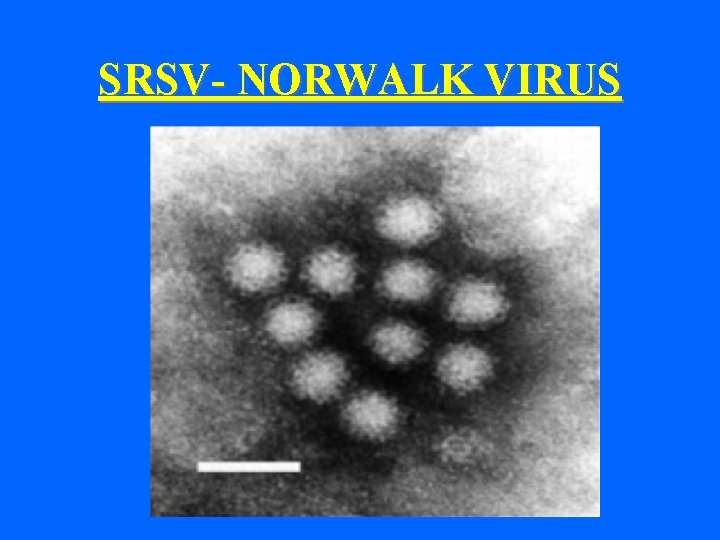
Morphology of Hu. CV- atypical • Atypical morphology • Smooth surface • Small Round Structured viruses E. g. - Norwalk-like viruses

SRSV- NORWALK VIRUS

CLINICAL FEATURES • Adults and Children • Usual incubation Period is <24 hours (ranges from 12 hrs. to 4 days) • Short duration of illness <3 days • Nausea, vomiting, fever, headache • Abdominal cramping • Watery diarrhea

Epidemiology-Noroviruses • Worldwide distribution • >23 million cases/year in the U. S. • Major cause of foodborne outbreaks of GE • Most people have had infections by age 4 years (by seroprevalence studies)

Spread of Norwalk virus A. Person-to-person Fecal-oral spread (stool/vomitus) B. Fecal contamination of food or water C. Spread through fomites?

Epidemiology-Noroviruses • Asymptomatic infections- seroconversion but asymptomatic shedding of virus • Low infective dose • Viral excretion during convalesence (up to 2 weeks) • Ability to survive in water chlorination at routine levels

Epidemiology of Outbreaks • Cruise ships, schools, nursing homes, etc. • Can involve infants and school-age children • Source usually is contaminated food and water (seafood-oyster and shellfish etc. )
![Diagnosis Human Caliciviruses Specimen stool vomitus environmental swabs not yet on foods Diagnosis- Human Caliciviruses • Specimen- stool , vomitus, environmental swabs, [not yet on foods]](https://slidetodoc.com/presentation_image/1d789a1d1d2908b904d41582e92b0710/image-60.jpg)
Diagnosis- Human Caliciviruses • Specimen- stool , vomitus, environmental swabs, [not yet on foods] • Immune EM • RT-PCR in state public health labs. • Serology for epidemiologic purposes

HUMAN ASTROVIRUS

ASTROVIRUS • Described in relation to an outbreak of gastroenteritis in 1975 • Detected by EM • Immunologically distinct from Human Caliciviruses • Belong to family Astroviridae • 8 human serotypes are known

ASTROVIRUS- structure • Small ss RNA virus • Non-enveloped • 27 -32 nm in size • Round with an unbroken, smooth surface • EM appearance of a 5 or 6 pointed star within smooth edge • Contain 3 structural proteins

ASTROVIRUS- EM STRUCTURE

ASTROVIRUS - Epidemiology • Worldwide • Mainly in children <7 years of age. • Transmission person-to-person via fecal-oral route • Outbreaks due to fecal contamination of sea-food or water

ASTROVIRUS - Clinical Features • Infants and children are most often affected • Short incubation period 1 -4 days • Nausea, vomiting, abdominal cramping and watery diarrhea • Constitutional symptoms-fever, malaise, headache

ASTROVIRUS - Diagnosis • EM (virus shed in stool in great numbers) • EIA • RT-PCR


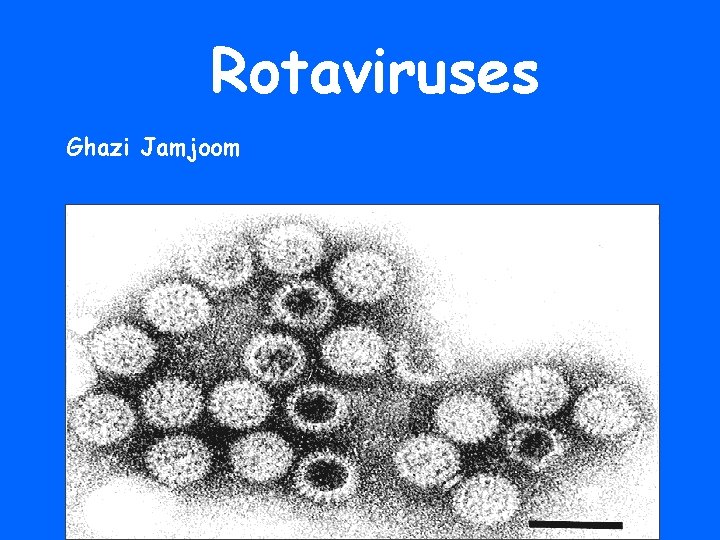
Rotaviruses Ghazi Jamjoom

• Twenty-five years ago, little was known about the causes of diarrhea, which kills an estimated 3 million infants and children worldwide every year. • Scientists knew that bacteria and parasites were implicated in only approximately 10 to 20 % of all cases of diarrhea. .

• In 1973, , researchers in Australia discovered a virus in infants with severe diarrhea and named it “rotavirus” for its wheel-like shape. • One year later, NIAID researchers were the first to identify rotavirus in the United States.

Rotavirus Biology • Rotaviruses belong to the family Reoviridae , genus Rotavirus. • They have a characteristic wheel-like appearance when viewed by electron microscopy. • Nonenveloped, double-shelled



Group A rotaviruses • Endemic worldwide (represents > 95% of currently identified strains in humans ) • The leading cause of severe diarrhea among infants and children. • Accounts for about half of the cases requiring hospitalization.

Group B rotavirus, • Also called adult diarrhea rotavirus or ADRV • Has caused major epidemics of severe diarrhea affecting thousands of persons of all ages in China.

Group C rotavirus • • Has been associated with rare and sporadic cases of diarrhea in children in many countries. • First outbreaks were reported from Japan and England

• Subgroups classification based upon neutralization epitopes of the outer capsid proteins, VP 4 and VP 7 Antigenic specificity of VP 7 G serotypes Antigenic specificity of VP 4 P serotypes Fourteen G serotypes and twenty one P serotypes have been detected in humans. Neutralization assays measure reactivity predominantly to VP 7 proteins.

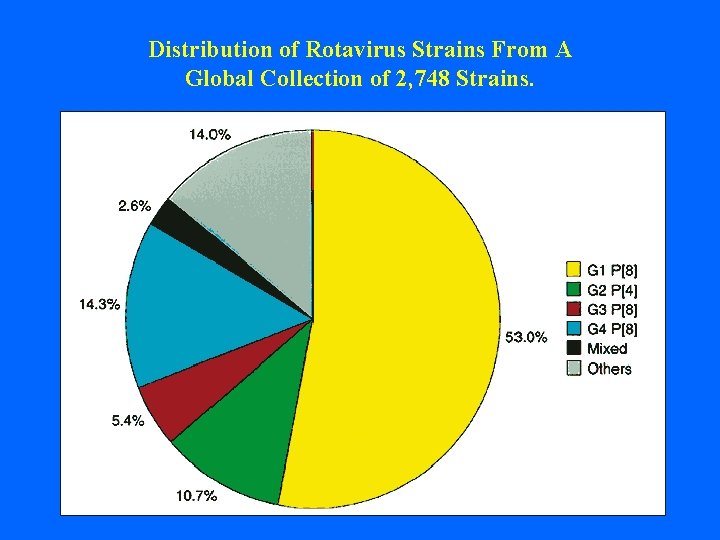
Distribution of Rotavirus Strains From A Global Collection of 2, 748 Strains.

Transmission • Rotavirus infection is very contagious. • Viral particles pass in the stool of infected persons before and after they have symptoms of the illness. • Spread is by the oral-fecal route

and tables) and is not killed by standard fectants. . . children forget to wash their hands often gh, especially before eating and after using the toilet. t Get infected d food handlers may contaminate foods that require ing and no further cooking, such as salads, fruits.

• The infective dose is presumed to be 10 -100 infectious viral particles. Because a person with rotavirus diarrhea often excretes large numbers of the virus (108 -1010 infectious particles/ml of feces), infection doses can be readily acquired.

• Asymptomatic rotavirus excretion has been well documented may play a role in perpetuating endemic disease. N. B some have reported low titers of virus in respiratory tract secretions and other body fluids. .

Epidemiology

Rotavirus is the single most important cause of life-threatening diarrhea in children younger than 2 years. • • Affects approximately 130 million infants and children worldwide. In the United States alone, rotavirus causes more than 3 million cases of childhood diarrhea each year, leading to an estimated 55, 000 to 100, 000 hospitalizations and 20 to 100 deaths. •

Who catch the infection ? • Humans of all ages are susceptible to rotavirus infection, although Children , premature infants, the elderly, and the immunocompromised are prone to more severe symptoms caused by infection with group A rotavirus.

• An infant’s first bout of diarrhea from rotavirus is the most severe , subsequent reinfections decrease in severity. These findings indicated that infants gradually develop partial immunity to the virus and that a vaccine might prevent the disease.

• Temporary lactose intolerance may occur. • NSP 4 protein may act in a toxin-like manner Neuronal alteration in water absorption Release of neuronal activators Calcium ion influx into enterocytes • Loss of the ability to absorb water net secretion of water and loss of ions watery diarrhea

Watery diarrhea dehydration (most commonly isotonic) and may lead to metabolic acidosis and death.

Symptoms and Signs • The incubation period ranges from 1 -3 days. • Symptoms often start with vomiting followed by 4 -8 days of diarrhea. • Some may have a slight rise in temperature.

Immunological Aspects • Immunoglobulin (Ig) A , in the lumen of the gut immunity to infection. • Actively or passively acquired antibodies (including antibodies in colostrum and mothers milk) lessen the severity of disease but does not consistently prevent reinfection. • Absence of antibody small amounts of virus infection and diarrhea.

• Infection in infants and small children is generally symptomatic. • In adults infection is usually asymptomatic. • Asymptomatic rotavirus infections are common in neonates because of passively acquired maternal immunity , breast feeding , and possible infection with less virulent strains

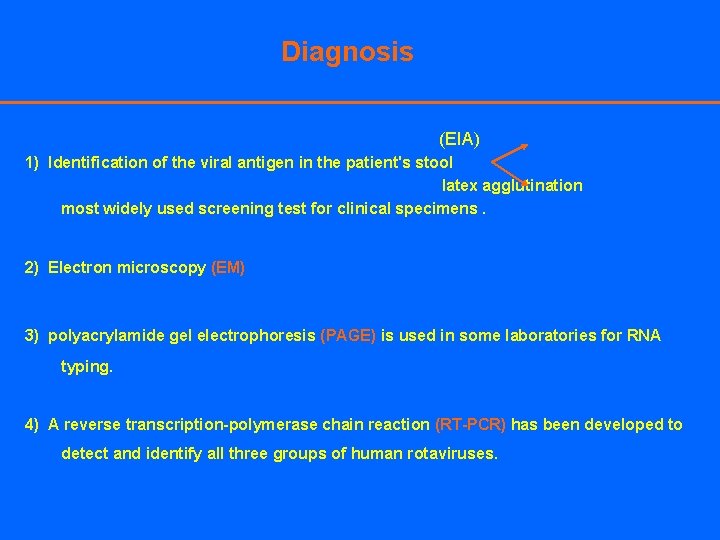
Diagnosis (EIA) 1) Identification of the viral antigen in the patient's stool latex agglutination most widely used screening test for clinical specimens. 2) Electron microscopy (EM) 3) polyacrylamide gel electrophoresis (PAGE) is used in some laboratories for RNA typing. 4) A reverse transcription-polymerase chain reaction (RT-PCR) has been developed to detect and identify all three groups of human rotaviruses.


• Serotypes can be identified using monoclonal antibodies against VP 7 and VP 4. • Neutralization is detected on tissue cultures as a CPE Antigenic specificity of VP 7 G serotypes Antigenic specificity of VP 4 P serotypes

Treatment • Treatment is nonspecific and consists of : 1) Oral rehydration therapy to prevent dehydration. 2) About one in 40 children with rotavirus gastroenteritis will require hospitalization for intravenous fluids. • For persons with healthy immune systems, rotavirus gastroenteritis is a self-limited illness, lasting for only a few days.

prevention • Even in the cleanest environments with the best hygiene, most children still become infected with rotavirus before age 4 or 5. • Total prevention of the spread of rotavirus is virtually impossible. • In hospitals health officials control rotavirus outbreaks by isolating infected patients and by ordering strict hand-washing procedures.

Rotavirus Vaccine

Rotavirus vaccine • Scientists knew that although many strains of rotavirus exist, only four cause the majority of diarrhea cases in young children in the United States. • Aiming for prevention : NIAID researchers developed a vaccine (RRV-TV) designed to protect against the four strains of rotavirus.

• During the 1970 s, NIAID scientists analyzed the genetic material of rotavirus, Identified two important proteins , VP 4 and VP 7 produced by the genes , and determined the function of these proteins. • Proteins on the surface of the virus were found to be critical for triggering an immune response in the body against rotavirus. • NIAID researchers focused on these proteins to develop a vaccine.

Tetravalent Oral Live-Attenuated Vaccine • The oral vaccine contains four different, live attenuated viral strains, serotypes ( 1, 2, 3, 4). • One strain (serotype 3) is an unmodified rhesus monkey rotavirus (RRV) which does not cause disease in humans • The other three are made by reassortment (genetic recombination) of that monkey RRV with three human rotaviruses of serotypes 1, 2, 4.

• Each reassortant vaccine strain contains 10 monkey RRV genes and the VP 7 gene for one serotype of the human rotavirus envelope proteins: VP-7 (serotype 1) , VP-7 (serotype 2) , VP-7 (serotype 4) • The combined vaccine provided comprehensive protection against the four serotypes (1, 2, 3, 4)

• Studies showed that high doses of the RRV-TV vaccine, designed to protect against four strains of rotavirus, were very effective in preventing severe, dehydrating rotavirus disease. • Breast-feeding did not interfere with the effectiveness of the rotavirus vaccine ensuring good nutrition in infants

• In August 1998, the first live attenuated rotavirus vaccine (Rotashield{registered} {Wyeth Lederle Vaccines and Pediatrics}) was approved for use in infants by the Food and Drug Administration. The Advisory Committee on Immunization Practices has recommended that this vaccine be given as a three-dose schedule to infants aged 2, 4, and 6 months.

• However, on July 15, 1999, the US Centers for Disease Control and Prevention (CDC) recommended that doctors stop giving the rotavirus vaccine to infants. • On October 22, 1999, the Advisory Committee on Immunization Practices voted to stop recommending the vaccine Why?

• Centers for Disease Control and Prevention (CDC) advisory committee received an overwhelming amount of data all indicating a strong association between ( rotavirus vaccine ) and bowel obstruction among some infants during the first one to two weeks following vaccination. • Apparently, many infants who received the rotavirus vaccine developed Intussception of the bowel within one to three weeks after receiving a dose or two of the vaccine.

• The risk of intussusception was increased 19 -fold in the first 3 to 7 days after vaccination and almost fourfold (3. 6) in the 8 to 14 days after vaccination (P<0. 0002).

• Children who have already received the vaccine and have not had problems do not appear to be at risk now. • In the meantime, research on better vaccines for rotavirus continues.

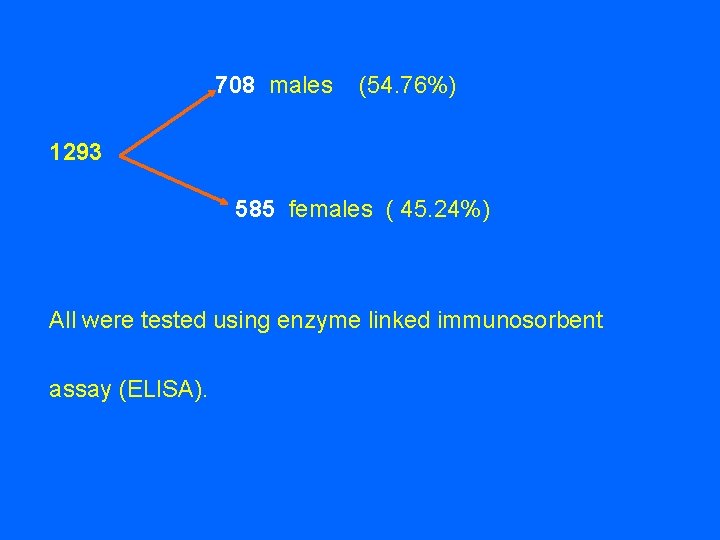
708 males 1293 (54. 76%) 585 females ( 45. 24%) All were tested using enzyme linked immunosorbent assay (ELISA).

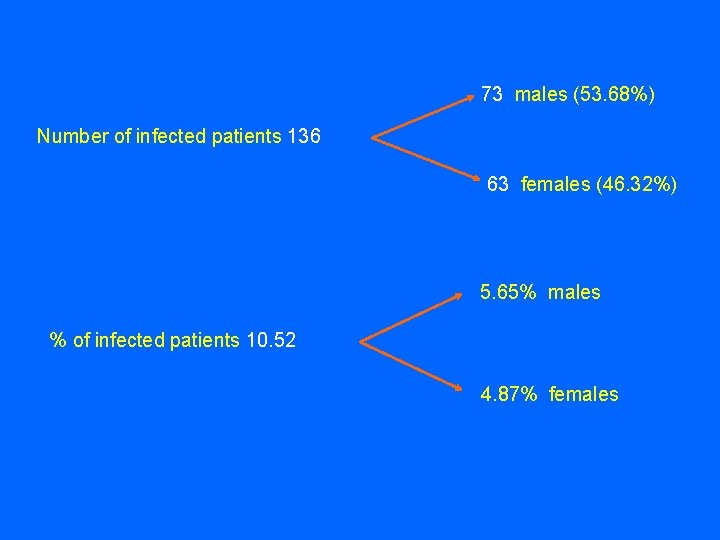
73 males (53. 68%) Number of infected patients 136 63 females (46. 32%) 5. 65% males % of infected patients 10. 52 4. 87% females

% of infected males = 10. 31 % % of infected females = 10. 77 %

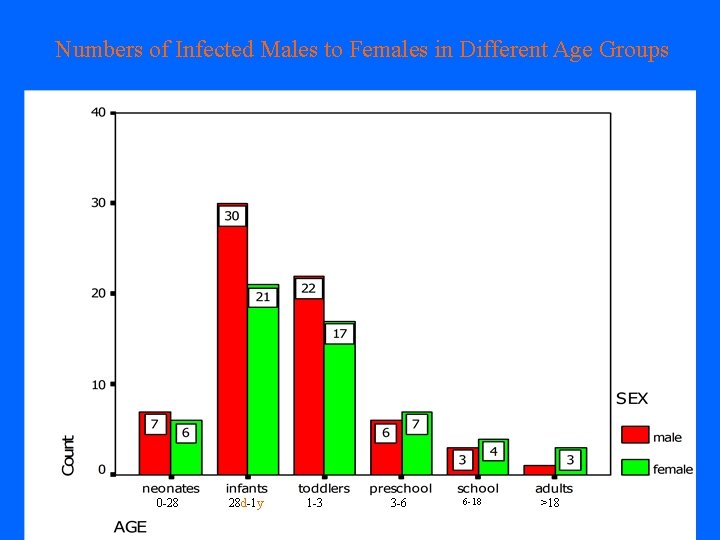
Numbers of Infected Males to Females in Different Age Groups 0 -28 28 d-1 y 1 -3 3 -6 6 -18 >18

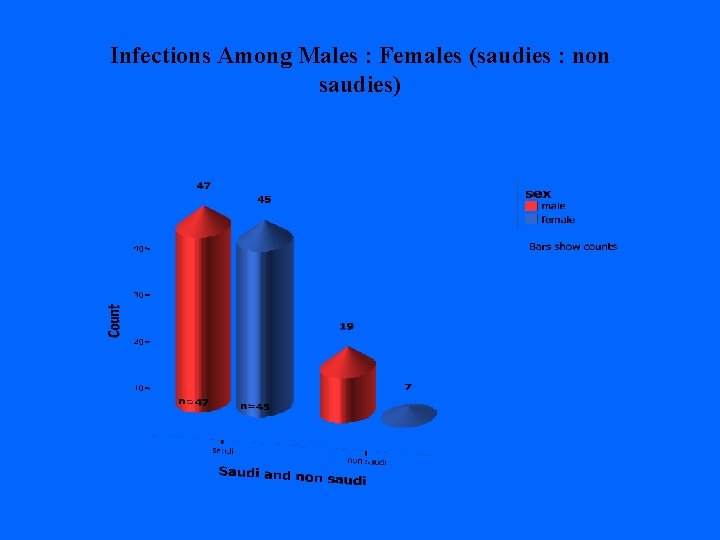
Infections Among Males : Females (saudies : non saudies)

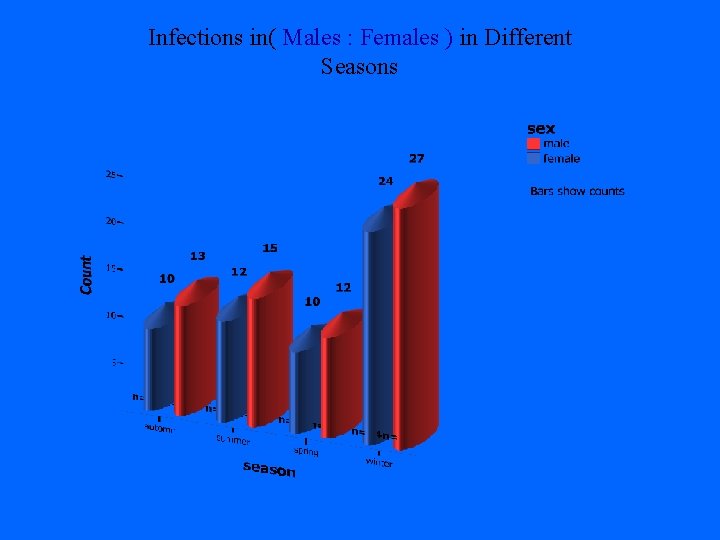
Infections in( Males : Females ) in Different Seasons

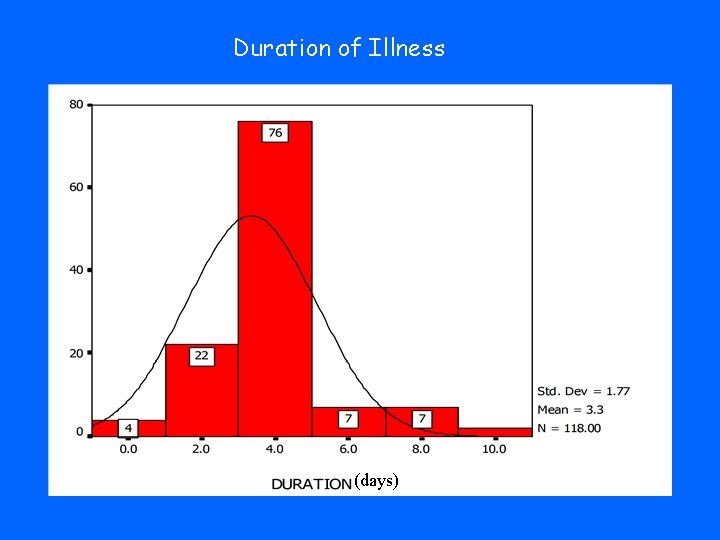
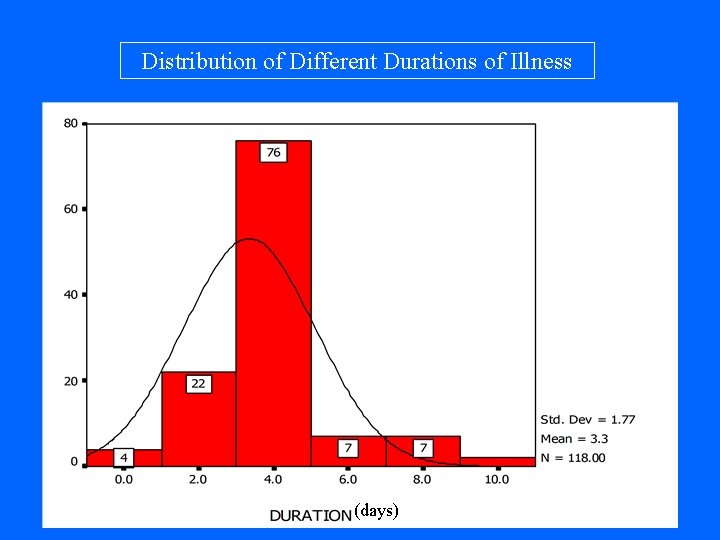
Duration of Illness (days)

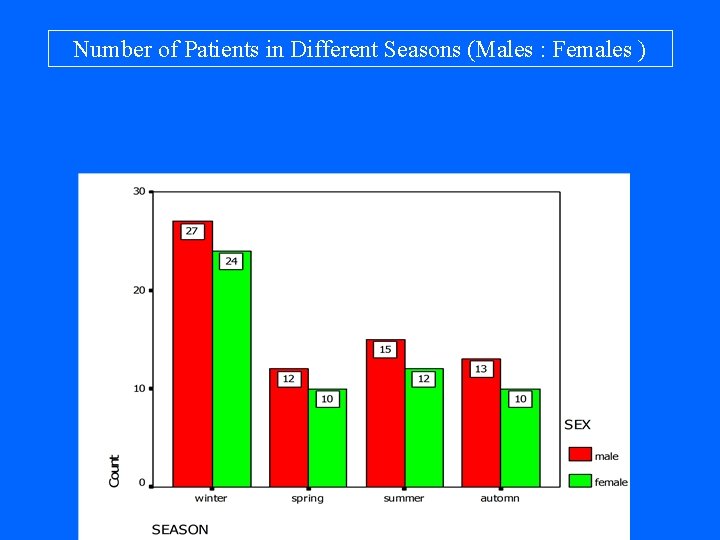
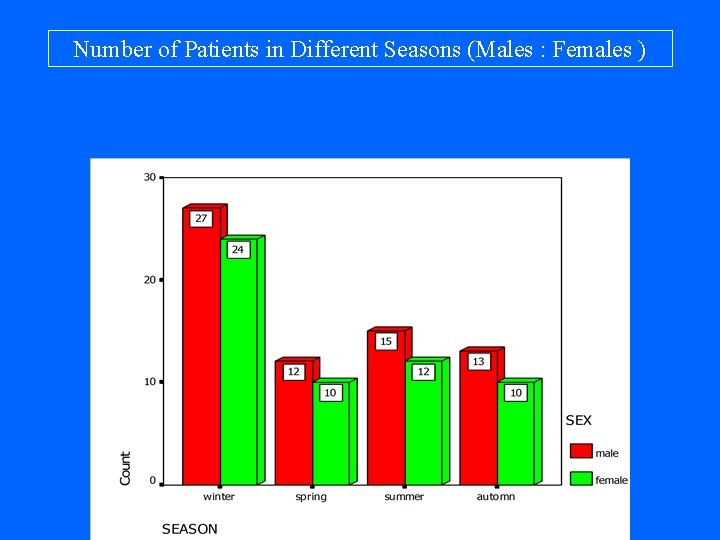
Number of Patients in Different Seasons (Males : Females )

Number of Patients in Different Seasons (Males : Females )

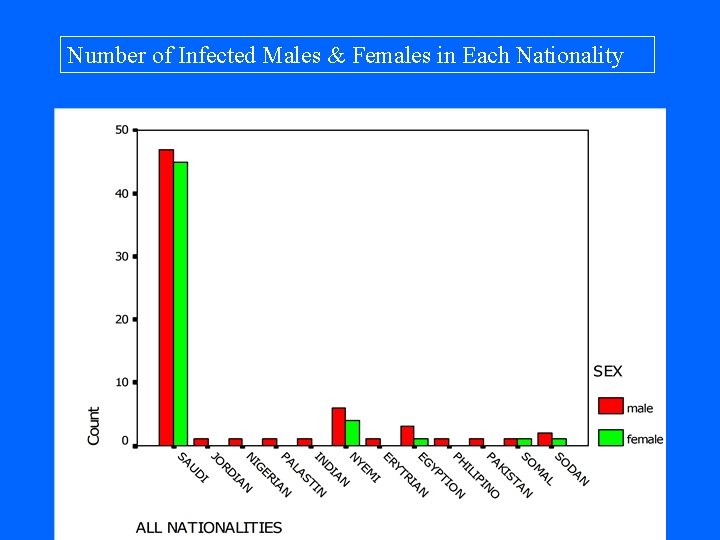
Number of Infected Males & Females in Each Nationality

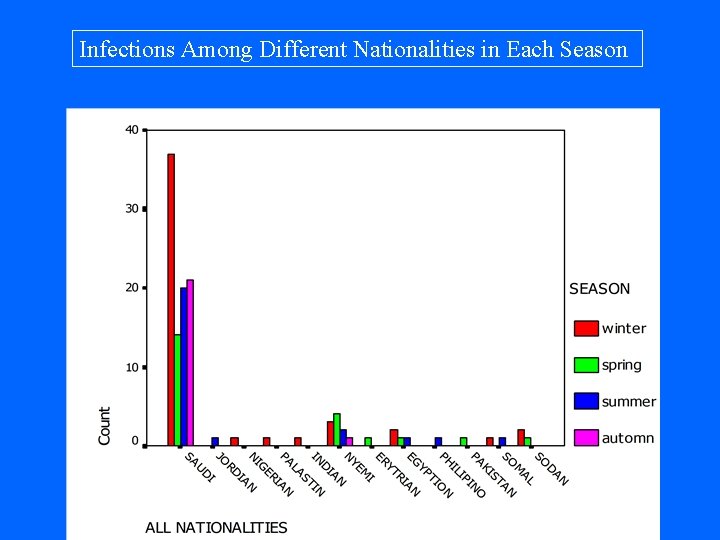
Infections Among Different Nationalities in Each Season

Distribution of Different Durations of Illness (days)