Virus Cell virus Types of genetic material in









- Slides: 27

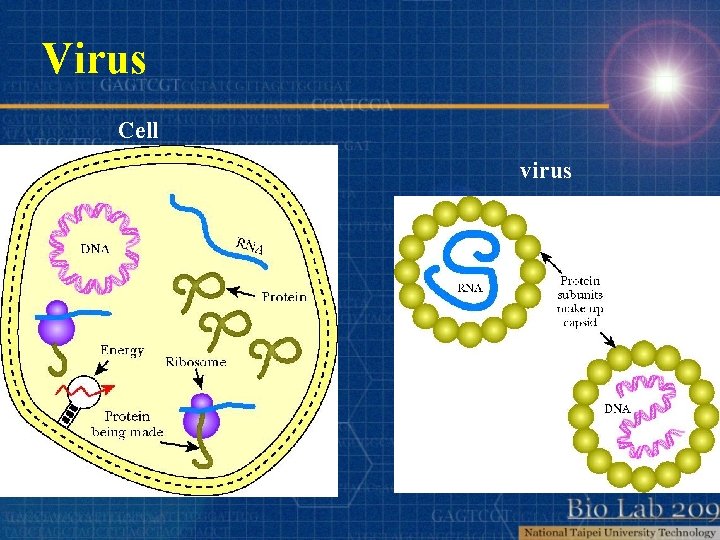
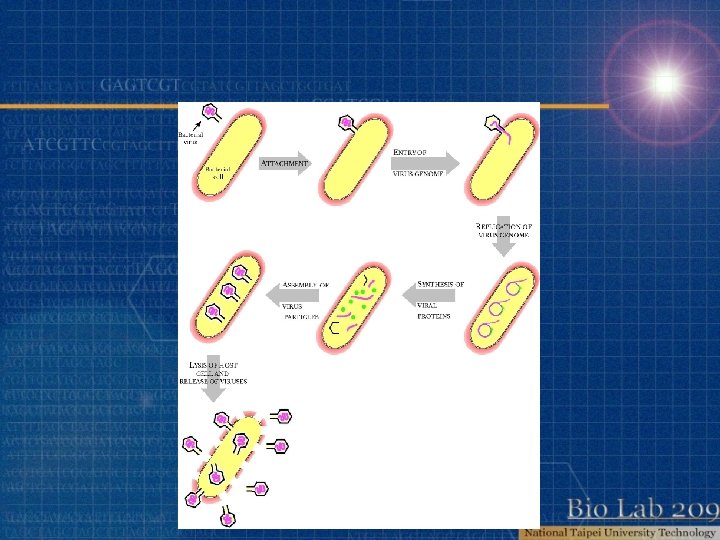
Virus Cell virus


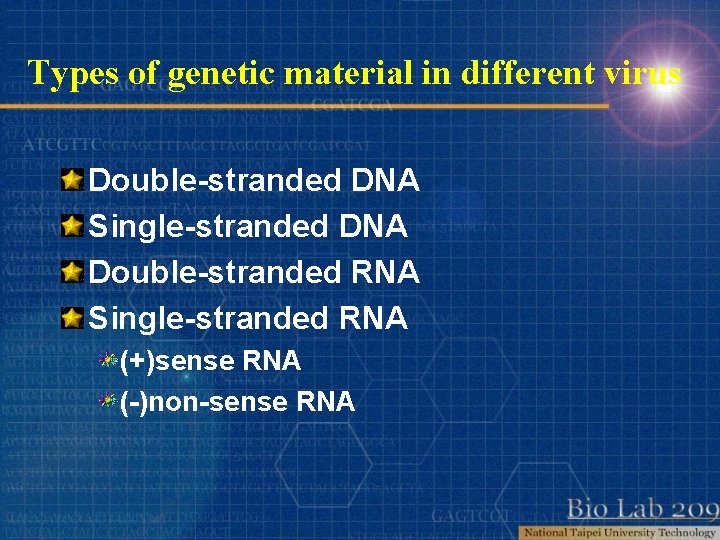
Types of genetic material in different virus Double-stranded DNA Single-stranded DNA Double-stranded RNA Single-stranded RNA (+)sense RNA (-)non-sense RNA

Types of genetic material in different virus


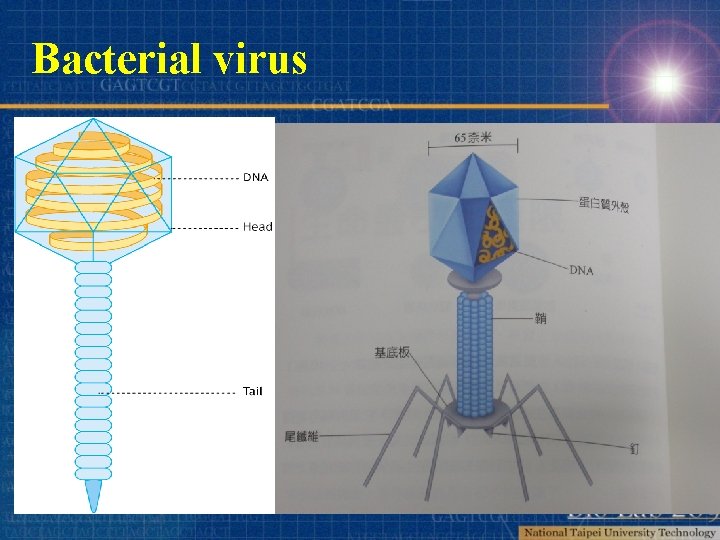
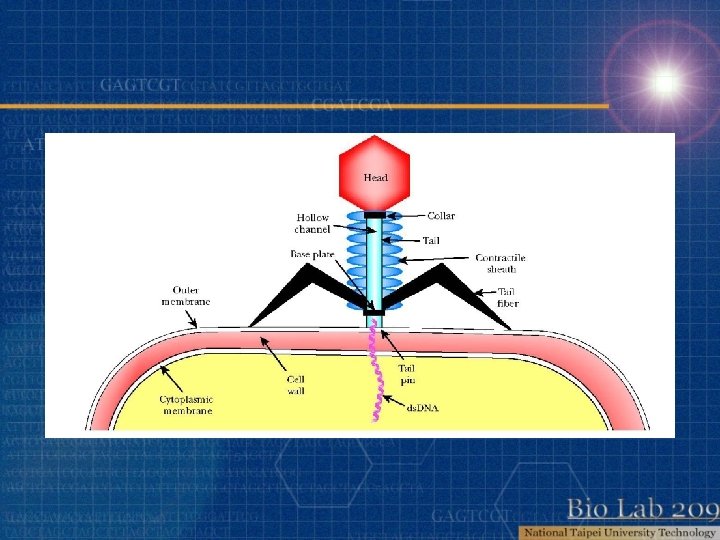
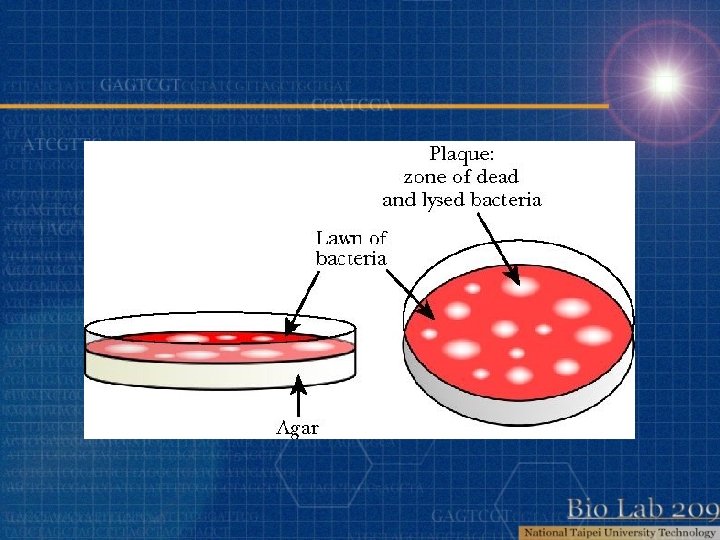
Bacterial virus


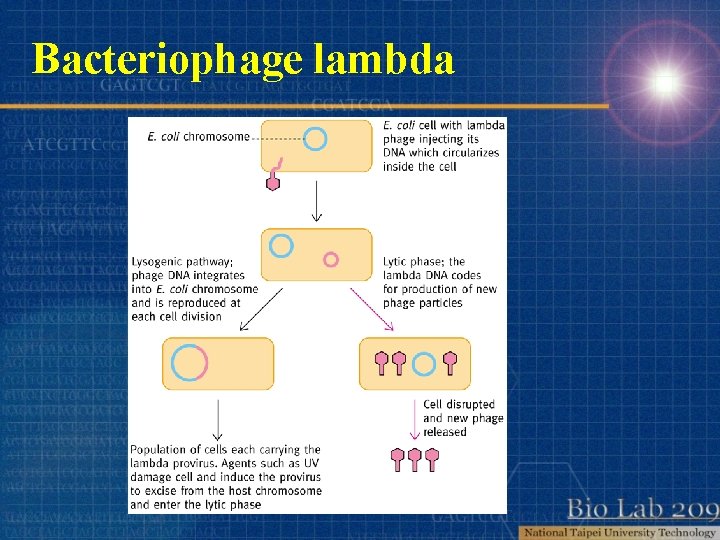
Bacteriophage lambda


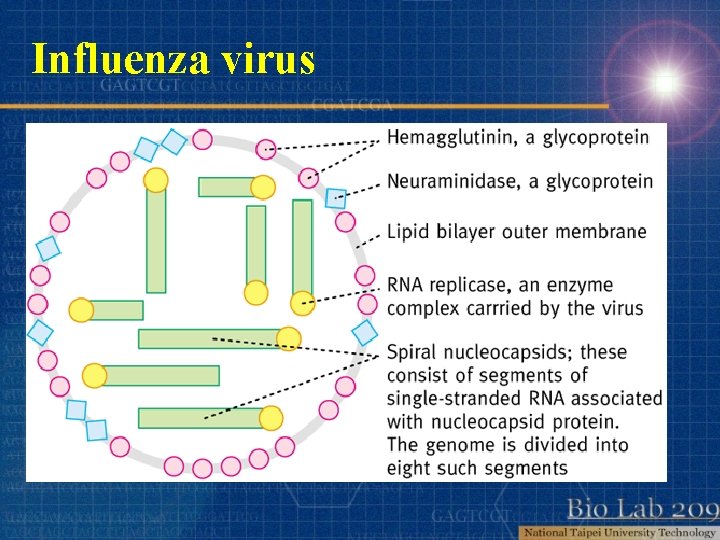
Influenza virus

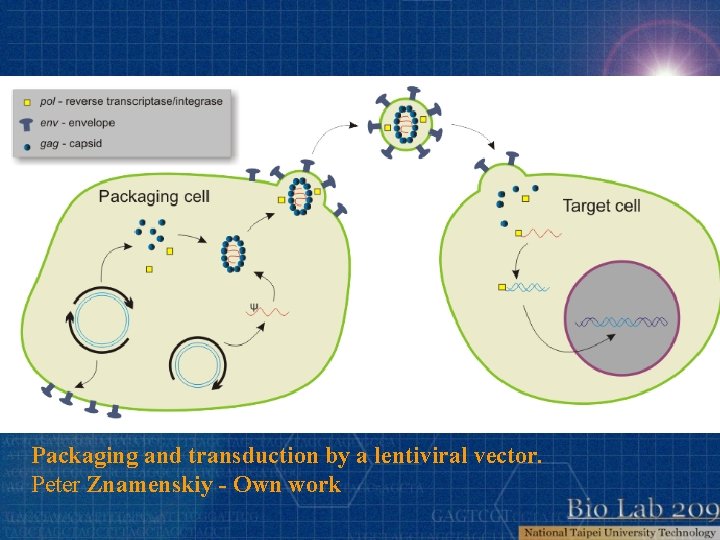
Packaging and transduction by a lentiviral vector. Peter Znamenskiy - Own work

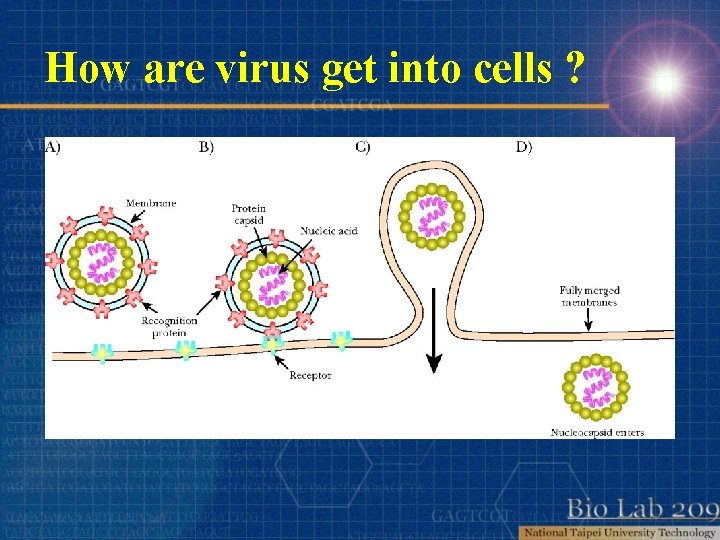
How are virus get into cells ?


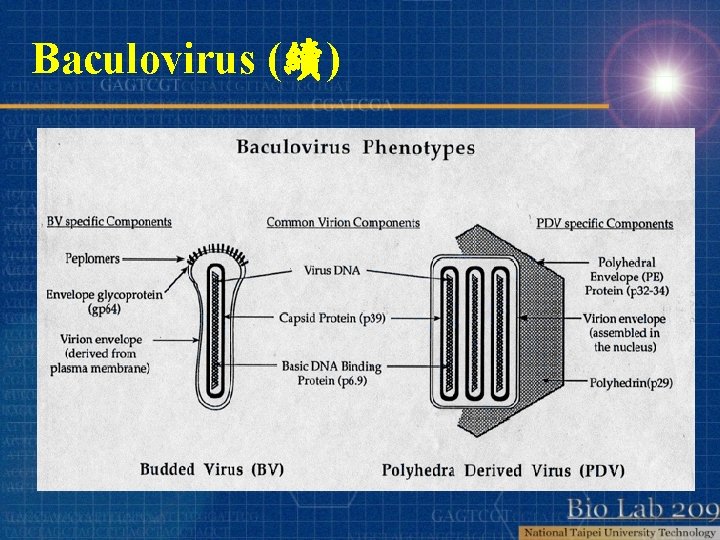
Baculovirus (續) Double-stranded , circular DNA 80 -200 kb size Accommodate an additional 100 kb or more of foreign DNA Two types of virions Polyhedra –derived virion (PDV) Budded virion (BV)

Baculovirus (續)

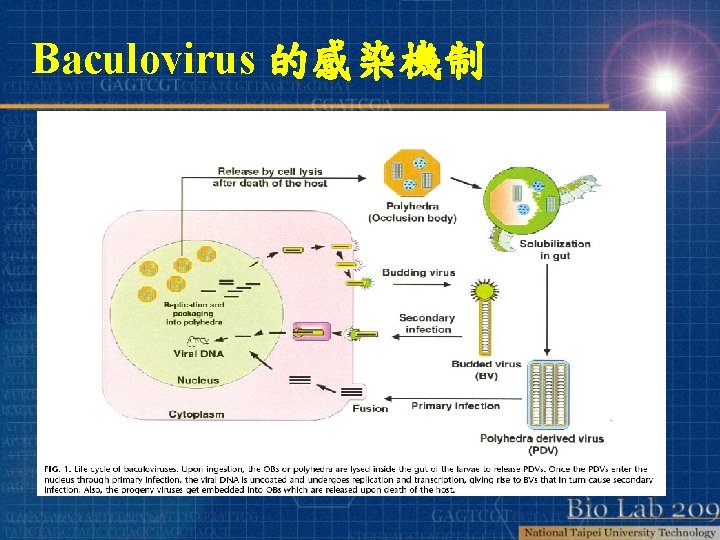
Baculovirus 的感染機制

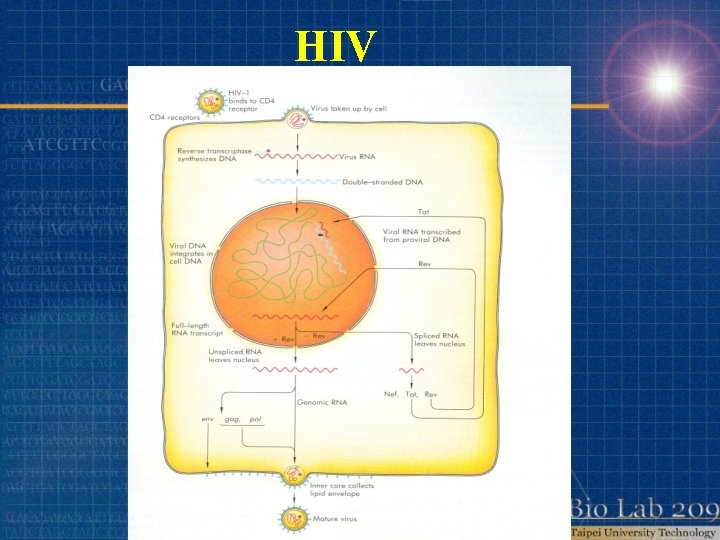
HIV

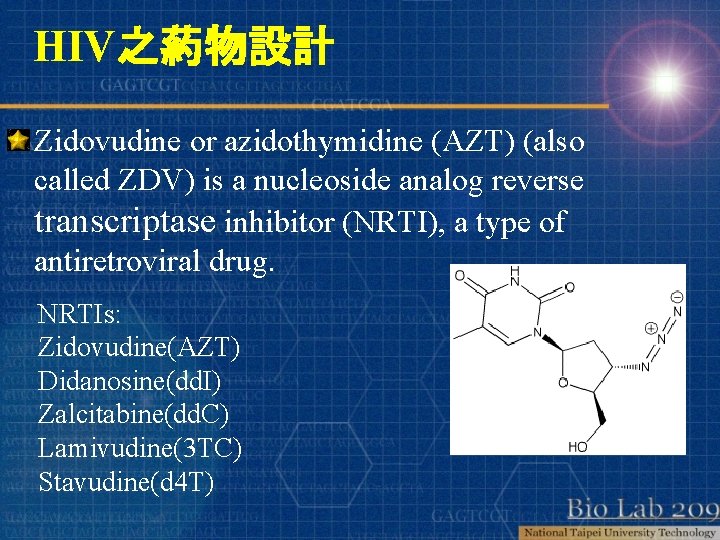
HIV之葯物設計 Zidovudine or azidothymidine (AZT) (also called ZDV) is a nucleoside analog reverse transcriptase inhibitor (NRTI), a type of antiretroviral drug. NRTIs: Zidovudine(AZT) Didanosine(dd. I) Zalcitabine(dd. C) Lamivudine(3 TC) Stavudine(d 4 T)

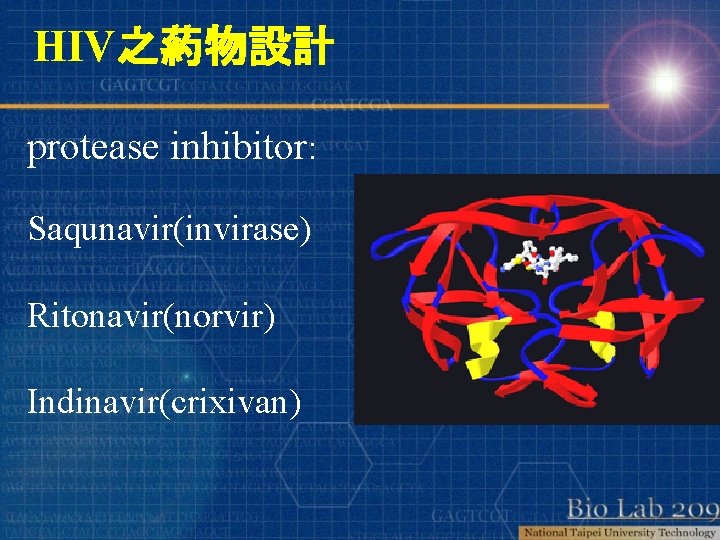
HIV之葯物設計 protease inhibitor: Saqunavir(invirase) Ritonavir(norvir) Indinavir(crixivan)

雞尾酒療法 Combinations of antiretrovirals create multiple obstacles to HIV replication to keep the number of offspring low and reduce the possibility of a superior mutation.

Food and Drug Administration( FDA,美國食品藥物檢驗局) The drug advertising regulation contains two key requirements. Under most circumstances, a company may only advertise a drug for the specific indication or medical use for which it was approved. Also, an advertisement must contain "fair balance" between the benefits and risks of a drug.

Clinical trial Pre-clinical studies in vitro (test tube) and in vivo (animal) experiments preliminary efficacy, toxicity and pharmacokinetic information Phase 0 Phase III approved Phase IV: Post Marketing Surveillance Trial

Clinical trial Pre-clinical studies Phase 0 2006 Distinctive features of Phase 0 trials include the administration of single subtherapeutic doses of the study drug to a small number of subjects (10 to 15) to gather preliminary data on the agent's pharmacokinetics (how the body processes the drug) and pharmacodynamics (how the drug works in the body).

Clinical trial Pre-clinical studies Phase 0 Phase I Normally, a small (20 -50) group of healthy volunteers will be selected. This phase includes trials designed to assess the safety, tolerability, pharmacokinetics, and pharmacodynamics of a drug. Phase III

Clinical trial Pre-clinical studies Phase 0 Phase II trials are performed on larger groups (20 -300) and are designed to assess how well the drug works, as well as to continue Phase I safety assessments in a larger group of volunteers and patients. Phase III

Clinical trial Pre-clinical studies Phase 0 Phase III studies are randomized controlled multicenter trials on large patient groups (300– 3, 000 or more depending upon the disease/medical condition studied) and are aimed at being the definitive assessment of how effective the drug is, in comparison with current 'gold standard' treatment. Because of their size and comparatively long duration, Phase III trials are the most expensive, time-consuming and difficult trials to design and run. approved

Polioviurs Naked virion (+)single-strand RNA Producing an RNA replicase Polyprotein