Virtual Data copper oxalate ppt lab Experiment 1
Virtual Data copper oxalate ppt lab Experiment #1: How can you experimentally determine the coefficients of a balanced chemical equation?
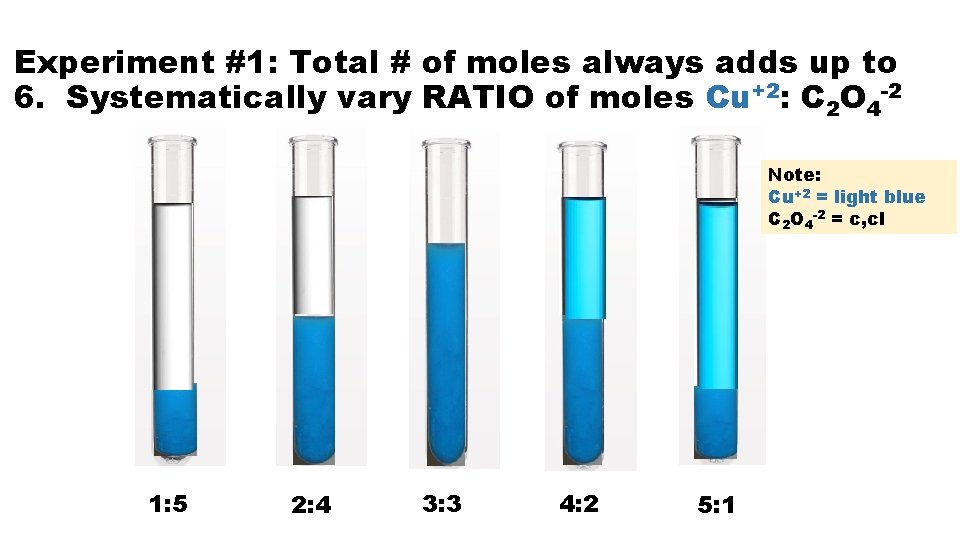
Experiment #1: Total # of moles always adds up to 6. Systematically vary RATIO of moles Cu+2: C 2 O 4 -2 Note: Cu+2 = light blue C 2 O 4 -2 = c, cl 1: 5 2: 4 3: 3 4: 2 5: 1
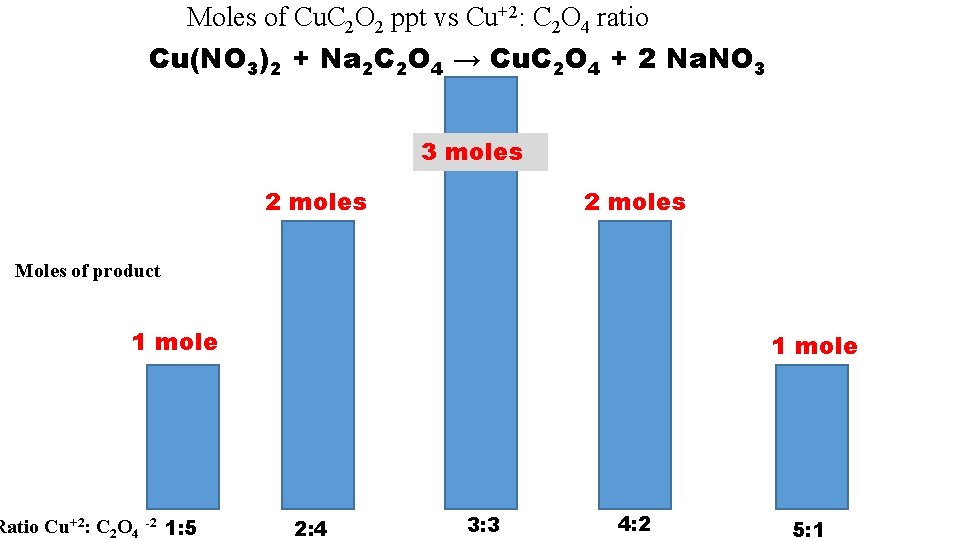
Moles of Cu. C 2 O 2 ppt vs Cu+2: C 2 O 4 ratio Cu(NO 3)2 + Na 2 C 2 O 4 → Cu. C 2 O 4 + 2 Na. NO 3 3 moles 2 moles Moles of product 1 mole Ratio Cu+2: C 2 O 4 -2 1: 5 1 mole 2: 4 3: 3 4: 2 5: 1
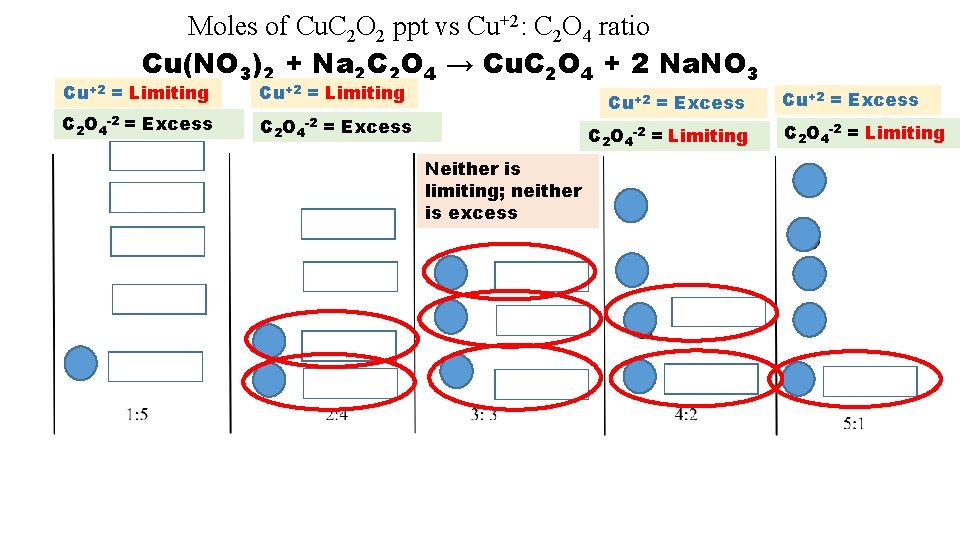
Moles of Cu. C 2 O 2 ppt vs Cu+2: C 2 O 4 ratio Cu(NO 3)2 + Na 2 C 2 O 4 → Cu. C 2 O 4 + 2 Na. NO 3 Cu+2 = Limiting C 2 O 4 -2 = Excess Cu+2 = Excess C 2 O 4 -2 = Limiting Neither is limiting; neither is excess Cu+2 = Excess C 2 O 4 -2 = Limiting
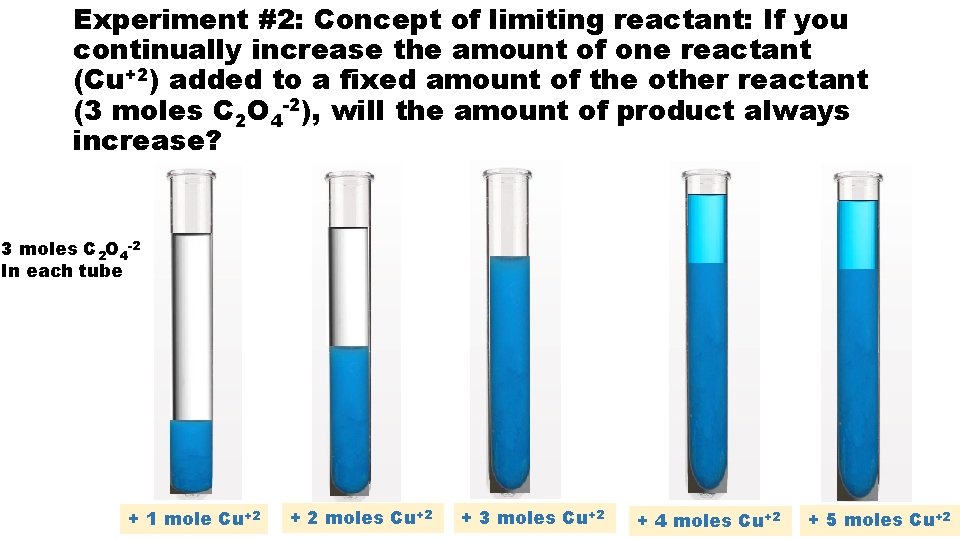
Experiment #2: Concept of limiting reactant: If you continually increase the amount of one reactant (Cu+2) added to a fixed amount of the other reactant (3 moles C 2 O 4 -2), will the amount of product always increase? 3 moles C 2 O 4 -2 In each tube + 1 mole Cu+2 + 2 moles Cu+2 + 3 moles Cu+2 + 4 moles Cu+2 + 5 moles Cu+2

Moles of Cu. C 2 O 2 ppt vs moles Cu. Cl 2 (+ 3 moles Na 2 C 2 O 4) Cu(NO 3)2 + Na 2 C 2 O 4 → Cu. C 2 O 4 + 2 Na. NO 3 3 moles 2 moles 3 moles Moles of product 1 mole Moles of Cu. Cl 2 1 2 3 4 5

Moles of Cu. C 2 O 2 ppt vs moles Cu. Cl 2 (+ 3 moles Na 2 C 2 O 4) Cu(NO 3)2 + Na 2 C 2 O 4 → Cu. C 2 O 4 + 2 Na. NO 3 Neither is limiting; neither is excess Cu+2 limiting C 2 O 4 excess Cu+2 excess C 2 O 4 limiting
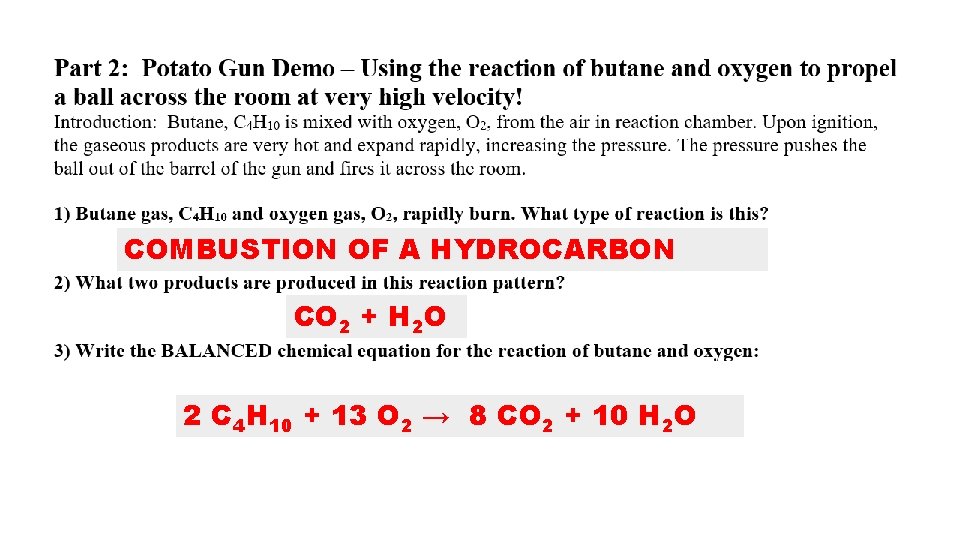
COMBUSTION OF A HYDROCARBON CO 2 + H 2 O 2 C 4 H 10 + 13 O 2 → 8 CO 2 + 10 H 2 O
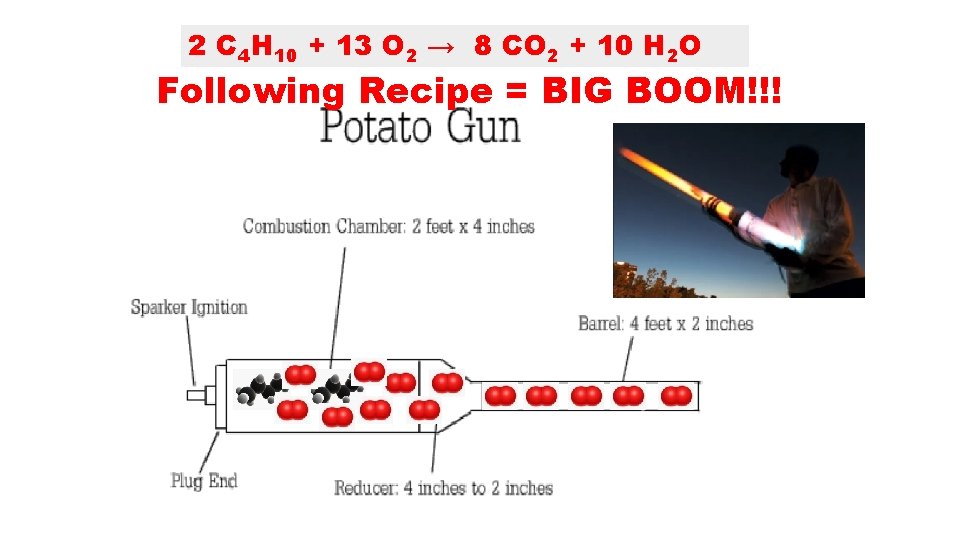
2 C 4 H 10 + 13 O 2 → 8 CO 2 + 10 H 2 O Following Recipe = BIG BOOM!!!
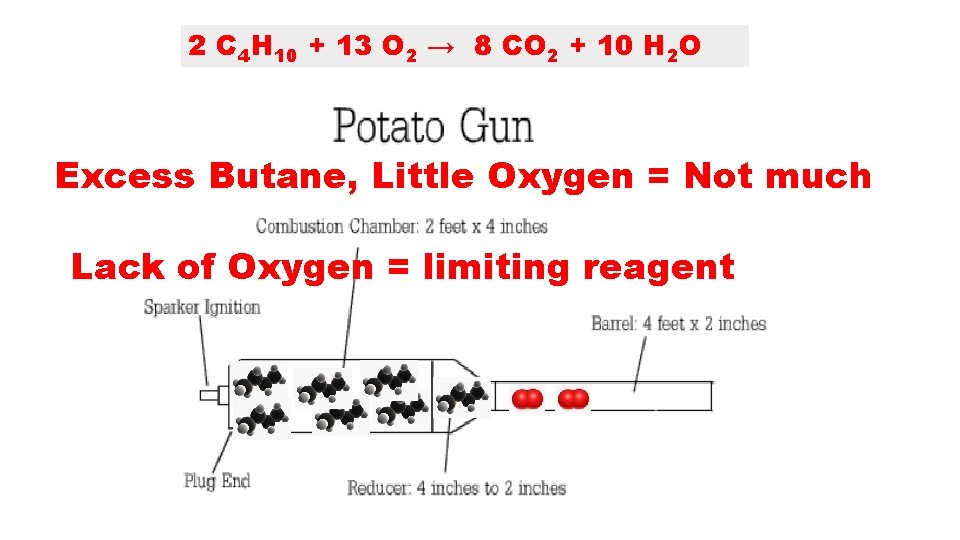
2 C 4 H 10 + 13 O 2 → 8 CO 2 + 10 H 2 O Excess Butane, Little Oxygen = Not much Lack of Oxygen = limiting reagent
- Slides: 10