Virologie Universit Paris 6 A Derrache Dr A












- Slides: 29

Virologie • Université Paris 6 – – – A Derrache Dr A Maiga Dr G Carcelain Dr AG Marcelin Pr V Calvez • Université Paris 7 – Dr D Descamps – Dr G Peytavin • Université Bordeaux 2 – Dr B Masquelier

Virologie • Diagnostic – Tests sérologiques – Diagnostic chez l’enfant • Suivi du traitement – Mesure de la charge virale • (détection de la virémie résiduelle) • Prise en charge de l’échec

Virologie • Diagnostic – Tests sérologiques

Tests ELISA

Tests rapides

Tests salivaires

Problèmes ? • Faux négatifs : exceptionnels • Faux positifs : très rares

Virologie • Diagnostic – Tests sérologiques – Diagnostic chez l’enfant

Avantages du papier buvard Possible 1 Étapes 2 inutiles 3 4


Virologie • Diagnostic – Tests sérologiques – Diagnostic chez l’enfant • Suivi du traitement – Mesure de la charge virale • (détection de la virémie résiduelle)

12 Échec à ABC+3 TC+fos. APV/r chez un patient naïf (SOLO) Viral load (copies/ml) 1 000 Pro : M 36 I RT : None Pro : M 36 I, M 46 I, I 50 V RT : M 184 V 100 000 Pro : M 36 I, M 46 I, I 50 V RT : L 74 V, M 184 V Pro : M 36 I, M 46 I, I 50 V RT : M 184 I 10 000 1 000 copies/ml 400 copies/ml * 100 50 copies/ml 10 0 20 40 60 80 100 120 140 160 180 Weeks on therapy * Switch to 908/r bid Sax et al

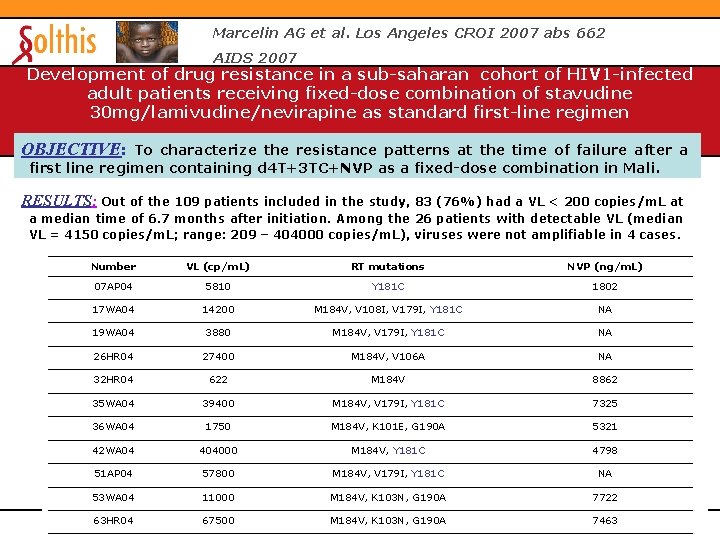
Marcelin AG et al. Los Angeles CROI 2007 abs 662 AIDS 2007 Development of drug resistance in a sub-saharan cohort of HIV 1 -infected adult patients receiving fixed-dose combination of stavudine 30 mg/lamivudine/nevirapine as standard first-line regimen OBJECTIVE: To characterize the resistance patterns at the time of failure after a first line regimen containing d 4 T+3 TC+NVP as a fixed-dose combination in Mali. RESULTS: Out of the 109 patients included in the study, 83 (76%) had a VL < 200 copies/m. L at a median time of 6. 7 months after initiation. Among the 26 patients with detectable VL (median VL = 4150 copies/m. L; range: 209 – 404000 copies/m. L), viruses were not amplifiable in 4 cases. Number VL (cp/m. L) RT mutations NVP (ng/m. L) 07 AP 04 5810 Y 181 C 1802 17 WA 04 14200 M 184 V, V 108 I, V 179 I, Y 181 C NA 19 WA 04 3880 M 184 V, V 179 I, Y 181 C NA 26 HR 04 27400 M 184 V, V 106 A NA 32 HR 04 622 M 184 V 8862 35 WA 04 39400 M 184 V, V 179 I, Y 181 C 7325 36 WA 04 1750 M 184 V, K 101 E, G 190 A 5321 42 WA 04 404000 M 184 V, Y 181 C 4798 51 AP 04 57800 M 184 V, V 179 I, Y 181 C NA 53 WA 04 11000 M 184 V, K 103 N, G 190 A 7722 63 HR 04 67500 M 184 V, K 103 N, G 190 A 7463

14

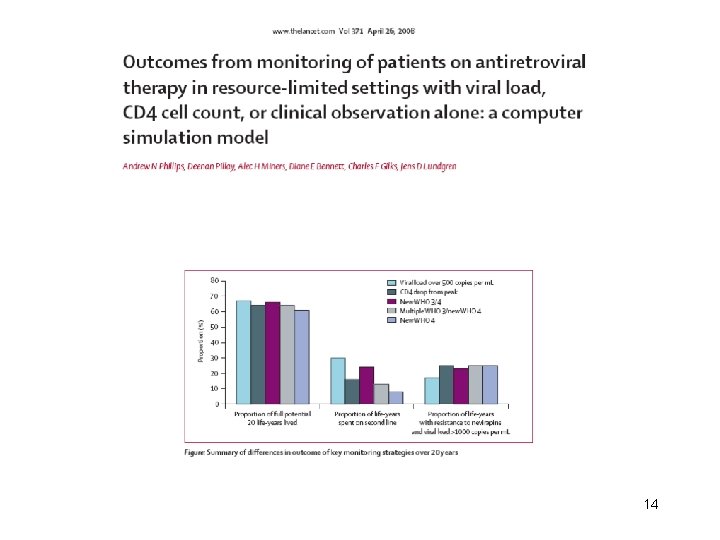
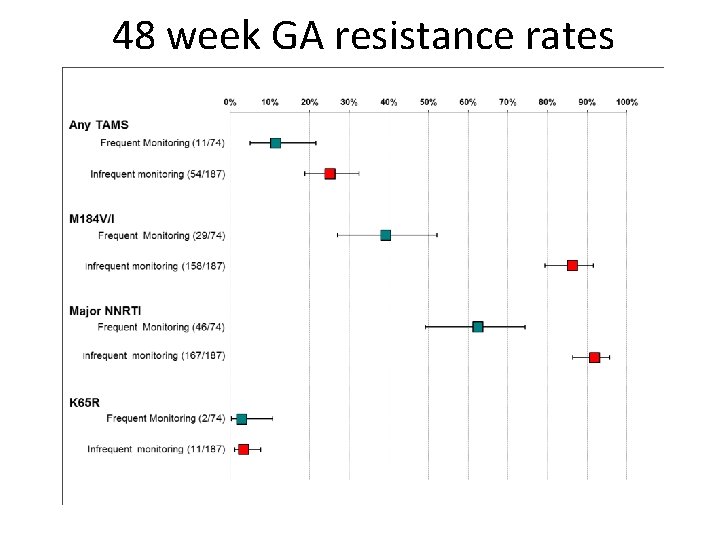
The intensity of virological monitoring is associated with resistance to first line HAART in HIV-1 infected adults receiving 1 st line therapy according to WHO guidelines: a systematic analysis of cohort and trial data Gupta RK, Hill A, Sawyer W, Cozzi-Lepri A, Phillips AN, von Wyl V, Yerly S, Gunthard HF, Gilks C, Pillay D

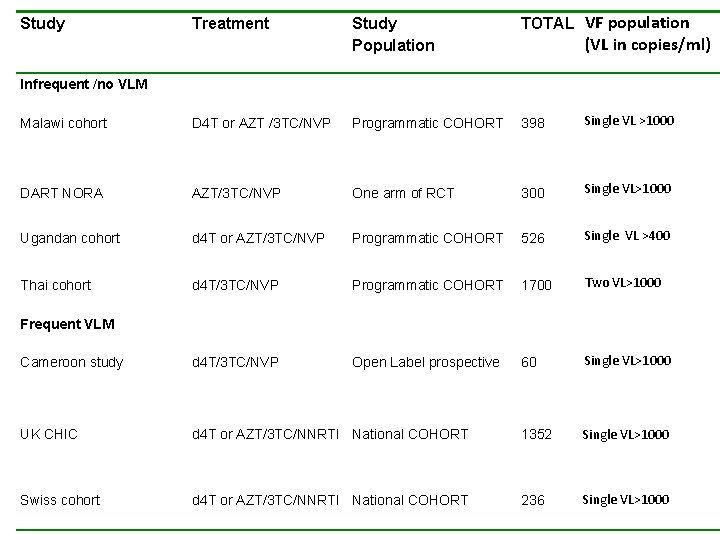
Study Treatment Study Population TOTAL VF population (VL in copies/ml) Infrequent /no VLM Malawi cohort D 4 T or AZT /3 TC/NVP Programmatic COHORT 398 Single VL >1000 DART NORA AZT/3 TC/NVP One arm of RCT 300 Single VL>1000 Ugandan cohort d 4 T or AZT/3 TC/NVP Programmatic COHORT 526 Single VL >400 Thai cohort d 4 T/3 TC/NVP Programmatic COHORT 1700 Two VL>1000 Cameroon study d 4 T/3 TC/NVP Open Label prospective 60 Single VL>1000 UK CHIC d 4 T or AZT/3 TC/NNRTI National COHORT 1352 Single VL>1000 Swiss cohort d 4 T or AZT/3 TC/NNRTI National COHORT 236 Single VL>1000 Frequent VLM

48 week GA resistance rates

48 week TOTAL resistance rates

Virologie • Diagnostic – Tests sérologiques – Diagnostic chez l’enfant • Suivi du traitement – Mesure de la charge virale • (détection de la virémie résiduelle) • Prise en charge de l’échec

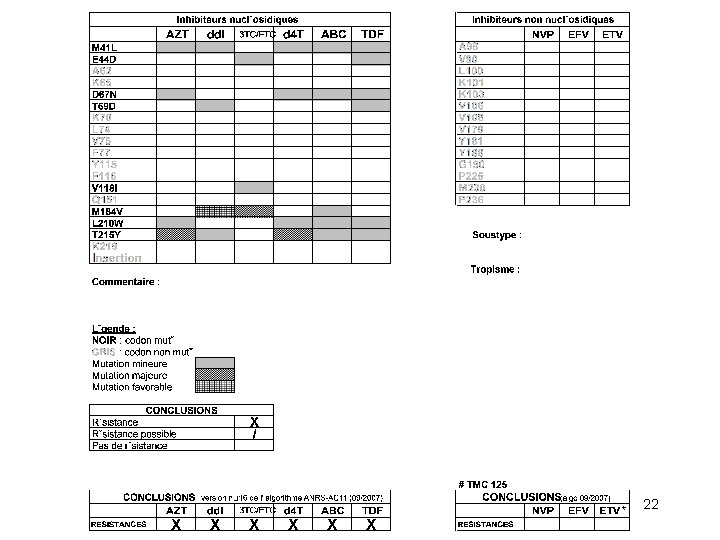
Résistance acquise aux NRTIs d 4 T, AZT TAMs 3 TC, FTC 184 ABC, dd. I, TDF 65, 74

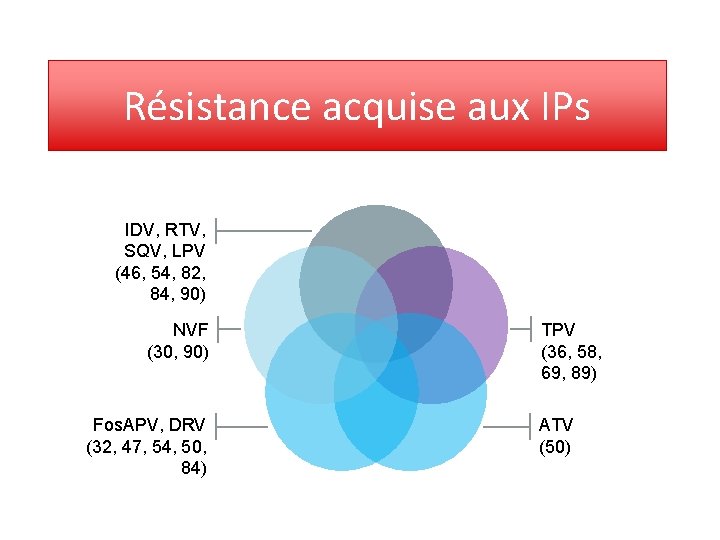
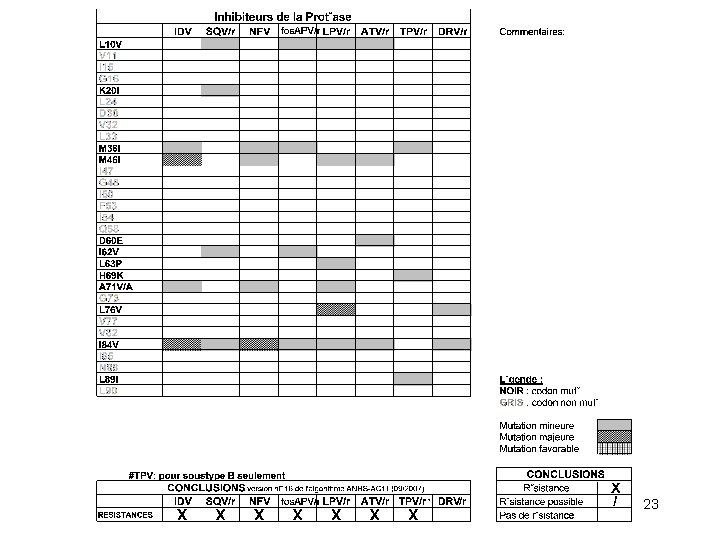
Résistance acquise aux IPs IDV, RTV, SQV, LPV (46, 54, 82, 84, 90) NVF (30, 90) Fos. APV, DRV (32, 47, 54, 50, 84) TPV (36, 58, 69, 89) ATV (50)

22

23


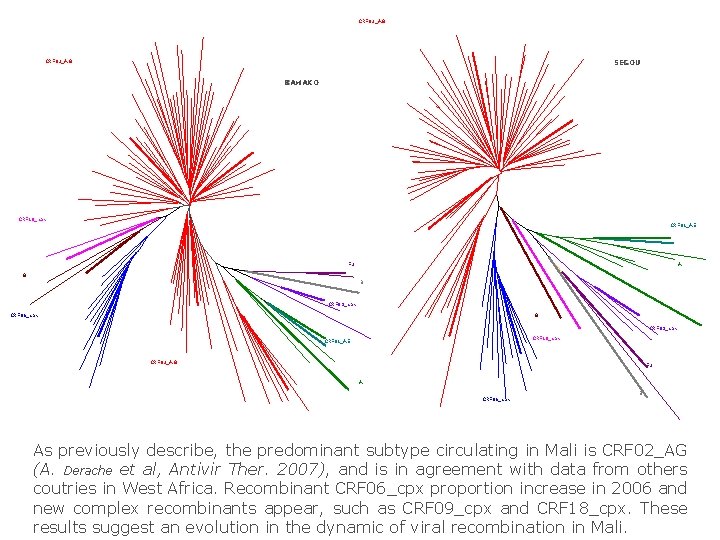
CRF 02_AG SEGOU CRF 02_AG BAMAKO CRF 18_cpx CRF 01_AE F 2 A G B CRF 09_cpx CRF 06_cpx G CRF 09_cpx CRF 18_cpx CRF 01_AE CRF 02_AG F 2 A B CRF 06_cpx As previously describe, the predominant subtype circulating in Mali is CRF 02_AG (A. Derache et al, Antivir Ther. 2007), and is in agreement with data from others coutries in West Africa. Recombinant CRF 06_cpx proportion increase in 2006 and new complex recombinants appear, such as CRF 09_cpx and CRF 18_cpx. These results suggest an evolution in the dynamic of viral recombination in Mali.


Prevalence of Resistance Mutations in Antiretroviral Naïve Chronically HIV-infected Patients in 2006/2007: a French Nationwide Study Diane Descamps, Brigitte Montes, Marie-Laure Chaix, Sophie Pakianather, Francis Barin, Georges Dos Santos, Anne Krivine, Constance Delaugerre, Jacques Izopet, Anne-Geneviève Marcelin, Anne Maillard, Laurence Morand-Joubert, Coralie Pallier, Jean-Christophe Plantier, Catherine Tamalet, Vincent Calvez, Bernard Masquelier, Françoise Brun-Vézinet 1, Dominique Costagliola on behalf the ANRS AC-11 Resistance Group.

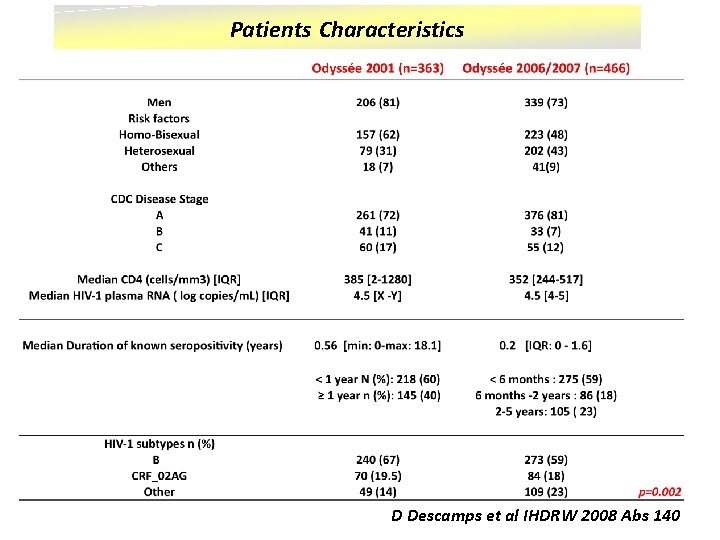
Patients Characteristics D Descamps et al IHDRW 2008 Abs 140

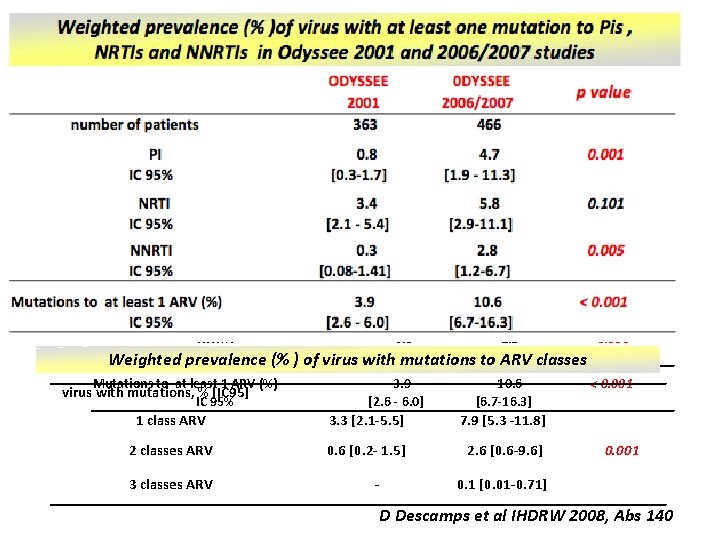
ODYSSEE 2001 363 0 DYSSEE 2006/2007 466 PI IC 95% 0. 8 [0. 3 -1. 7] 4. 7 [1. 9 - 11. 3] 0. 001 NRTI IC 95% 3. 4 [2. 1 - 5. 4] 5. 8 [2. 9 -11. 1] 0. 101 number of patients p value NNRTI 0. 3 2. 8 0. 005 IC 95% [1. 2 -6. 7] Weighted prevalence (% ) of virus [0. 08 -1. 41] with mutations to ARV classes Mutations to at least 1 ARV (%) IC 95% virus with mutations, % [IC 95] 3. 9 [2. 6 - 6. 0] 10. 6 [6. 7 -16. 3] 1 class ARV 3. 3 [2. 1 -5. 5] 7. 9 [5. 3 -11. 8] 2 classes ARV 0. 6 [0. 2 - 1. 5] 2. 6 [0. 6 -9. 6] 3 classes ARV - < 0. 001 0. 1 [0. 01 -0. 71] D Descamps et al IHDRW 2008, Abs 140
 Rotterdam university economics
Rotterdam university economics London universit
London universit Organigramme nanterre
Organigramme nanterre Universit
Universit Universit sherbrooke
Universit sherbrooke Eobe paris saclay
Eobe paris saclay Principles of casting
Principles of casting Comete nanterre
Comete nanterre Secouristes
Secouristes Monnaie de paris pessac
Monnaie de paris pessac Manuele
Manuele Romeo and juliet act v study guide
Romeo and juliet act v study guide Hrt wetter
Hrt wetter Romeo and juliet act 4 scene 1
Romeo and juliet act 4 scene 1 Novatest
Novatest Paris school of economics ape
Paris school of economics ape How tall is the eiffel tower in paris france
How tall is the eiffel tower in paris france Je traverse tout paris sans sortir de mon lit qui suis-je
Je traverse tout paris sans sortir de mon lit qui suis-je Calle de parís, día lluvioso
Calle de parís, día lluvioso Paglalakbay ni rizal sa espanya
Paglalakbay ni rizal sa espanya Le pantheon facts
Le pantheon facts The judgment of paris
The judgment of paris Paris saclay
Paris saclay Paris japonica
Paris japonica Ip web nanterre
Ip web nanterre Serge poliakoff xvi
Serge poliakoff xvi Quilles de huit paris
Quilles de huit paris Catherine tourtier
Catherine tourtier Von paris
Von paris Paris probe proves palace innocent
Paris probe proves palace innocent