Viral Hepatitis in Infants and Children Ricardo A














- Slides: 40

Viral Hepatitis in Infants and Children Ricardo A. Caicedo, M. D. Pediatric Gastroenterology and Nutrition Wake Forest University Baptist Medical Center

Learning Objectives • Recognize the clinical manifestations of viral hepatitis in the pediatric population • Understand the natural history of hepatotropic viral infections in children • Become familiar with the diagnosis and management of pediatric viral hepatitis

Topics • • Hepatotropic Viruses Acute vs. Fulminant vs. Chronic Hepatitis A virus Hepatitis B virus – Diagnosis – Natural history – Immunoprophylaxis/management • Hepatitis C virus – Diagnosis – Natural history – Management • Other viral agents www. microscopyu. com

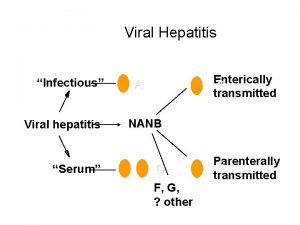
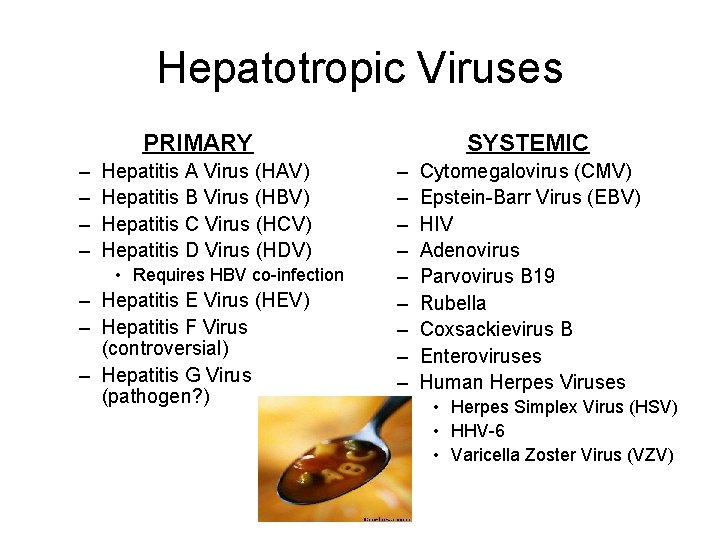
Hepatotropic Viruses PRIMARY – – Hepatitis A Virus (HAV) Hepatitis B Virus (HBV) Hepatitis C Virus (HCV) Hepatitis D Virus (HDV) • Requires HBV co-infection – Hepatitis E Virus (HEV) – Hepatitis F Virus (controversial) – Hepatitis G Virus (pathogen? ) SYSTEMIC – – – – – Cytomegalovirus (CMV) Epstein-Barr Virus (EBV) HIV Adenovirus Parvovirus B 19 Rubella Coxsackievirus B Enteroviruses Human Herpes Viruses • Herpes Simplex Virus (HSV) • HHV-6 • Varicella Zoster Virus (VZV)

Acute Viral Hepatitis • Acute hepatocellular injury/inflammation – Reflected by elevated transaminases (AST or SGOT, ALT or SGPT) – Clinical manifestations often include fever, malaise, jaundice, RUQ pain, nausea/vomiting • Typically self-limited and of short duration – Contrast with: chronic, fulminant • Causative agents – HAV (50% of cases in U. S. ), HEV – CMV, EBV, VZV

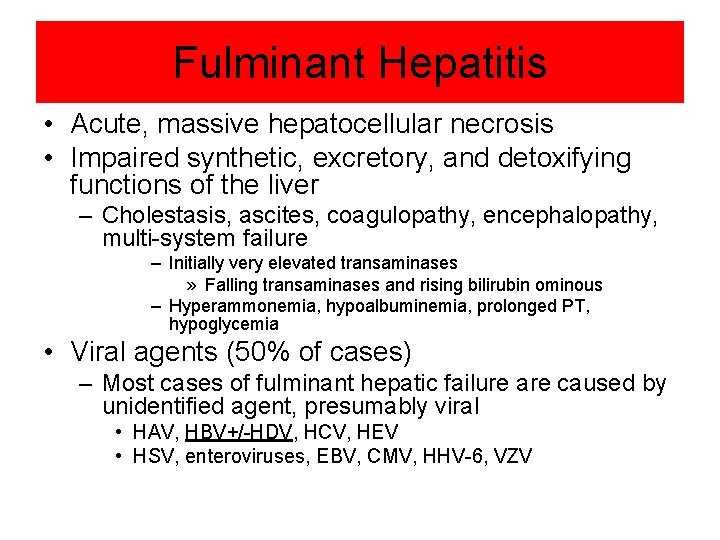
Fulminant Hepatitis • Acute, massive hepatocellular necrosis • Impaired synthetic, excretory, and detoxifying functions of the liver – Cholestasis, ascites, coagulopathy, encephalopathy, multi-system failure – Initially very elevated transaminases » Falling transaminases and rising bilirubin ominous – Hyperammonemia, hypoalbuminemia, prolonged PT, hypoglycemia • Viral agents (50% of cases) – Most cases of fulminant hepatic failure are caused by unidentified agent, presumably viral • HAV, HBV+/-HDV, HCV, HEV • HSV, enteroviruses, EBV, CMV, HHV-6, VZV

Chronic Hepatitis • Prolonged necroinflammatory process – Elevated transaminases for > 4 -6 months – Insidious clinical manifestations • Can include cholestasis (jaundice, pruritus), ascites, hypoalbuminemia, coagulopathy, encephalopathy • Can progress to fibrosis and then cirrhosis • Viral agents: HBV (+/- HDV), HCV • Other causes include autoimmune, metabolic disorders (Wilson’s, CF, alpha-1 antitrypsin deficiency), drug/toxin-mediated, idiopathic

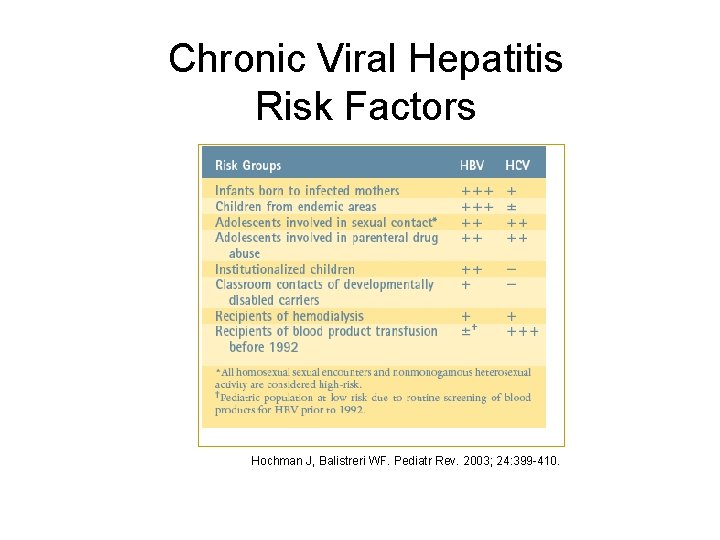
Chronic Viral Hepatitis Risk Factors Hochman J, Balistreri WF. Pediatr Rev. 2003; 24: 399 -410.

Hepatitis A Virus • Causes 33% of acute viral hepatitis in U. S. – NOT a cause of chronic hepatitis – rarely causes fulminant hepatitis (< 1% cases) • Can trigger autoimmune hepatitis in predisposed individuals • Epidemiologic factors – – Fecal-oral transmission Poor hygiene High population density Daycare centers and minor epidemics

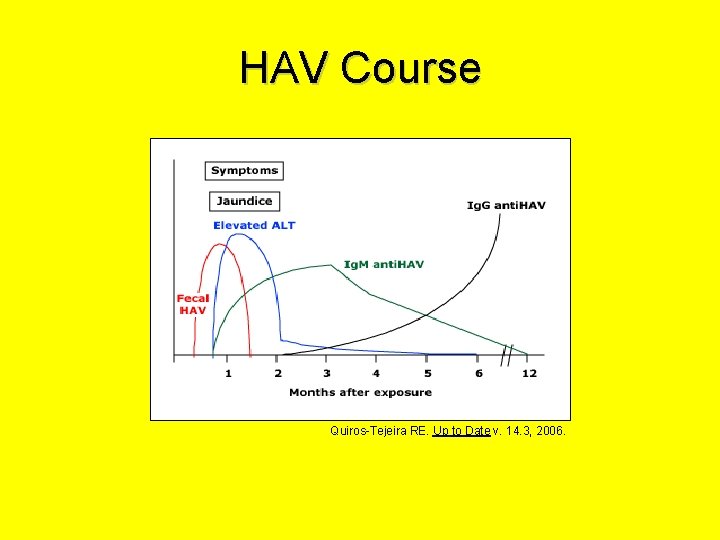
HAV • Acute, self-limited illness – Fever, malaise, anorexia, vomiting, nausea, abdominal pain, diarrhea – Elevated AST/ALT – Jaundice (conjugated hyperbilirubinemia) usually 1 wk after onset of symptoms – Duration • Age < 6 y: typically, <2 wks • Older children and adults can have prolonged course and often have hepatomegaly • Dx: serology – Anti-HAV Ig. M • Supportive care

HAV Course Quiros-Tejeira RE. Up to Date v. 14. 3, 2006.

HAV Vaccine • Prevents morbidity and mortality associated with HAV infection – Incidence of HAV in U. S. has decreased by 75% since vaccine introduced in 1997 • Because humans are the only known reservoir for HAV, universal immunization strategies could hypothetically eradicate HAV • AAP Recommendation, 2006 – All children age 12 -23 months should be immunized • Extended safety data supports incorporation of HAV vaccine into routine childhood immunization schedule

Recommended Standard Childhood Immunization Schedule, 2006 www. cdc. gov/nip/recs/child-schedule. htm

Hepatitis B Virus • Hepadnavirus – Double-stranded DNA • “A retrovirus in disguise” – Multiple genotypes and serotypes • Replication factory – Significant mutation rate • Can “escape” serological detection and/or vaccine • Triggers host immune attack on liver cells – T-cell-mediated hepatocellular lysis • Chronic infection results from ineffective immune response

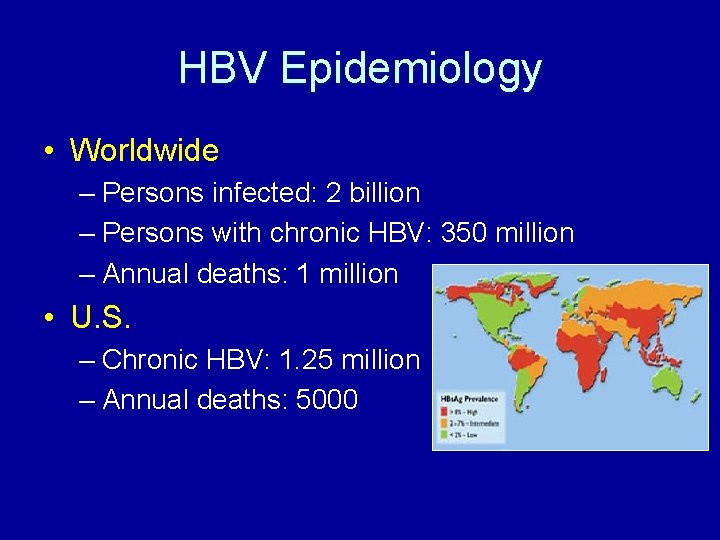
HBV Epidemiology • Worldwide – Persons infected: 2 billion – Persons with chronic HBV: 350 million – Annual deaths: 1 million • U. S. – Chronic HBV: 1. 25 million – Annual deaths: 5000

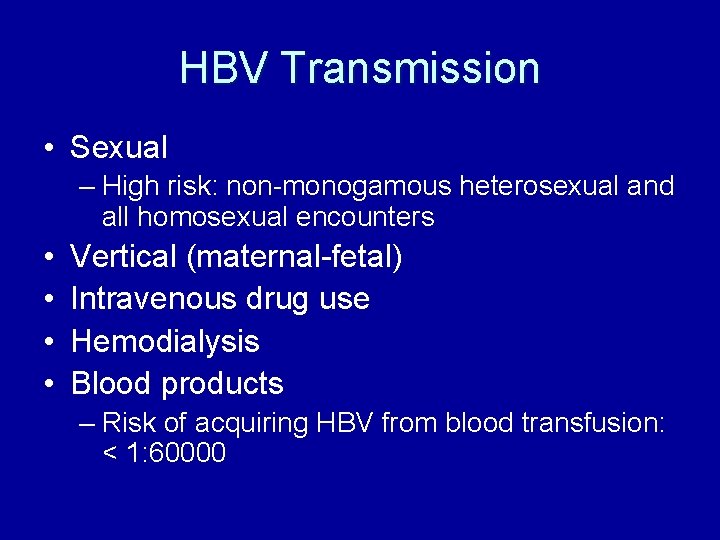
HBV Transmission • Sexual – High risk: non-monogamous heterosexual and all homosexual encounters • • Vertical (maternal-fetal) Intravenous drug use Hemodialysis Blood products – Risk of acquiring HBV from blood transfusion: < 1: 60000

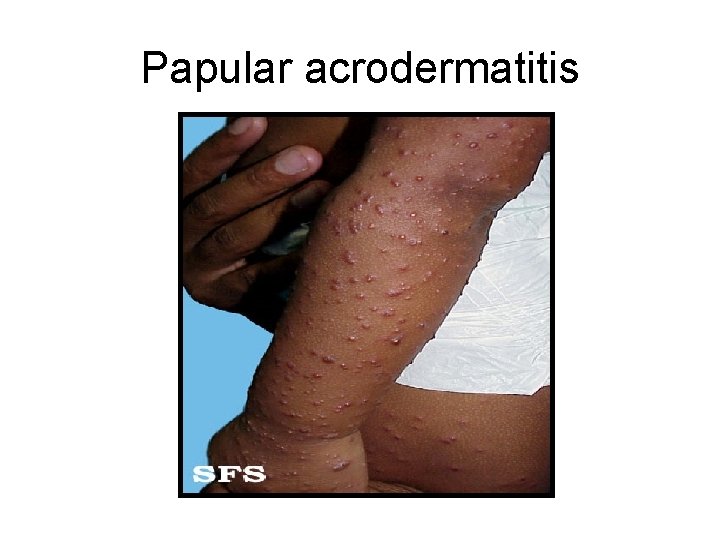
HBV Manifestations • Incubation period: 45 -160 d • Prodromal “flu-like” illness – Malaise, fatigue, anorexia, nausea, fever • Jaundice – Cholestasis • Elevated AST/ALT • Less common – Fulminant hepatitis: coagulopathy, encephalopathy • 1 -5% of HBV cases – Glomerulonephritis, arthritis – Papular acrodermatitis (Giannotti-Crosti)

Papular acrodermatitis

Screening for HBV • TESTS: HBs. Ag and anti-HBs • Adolescents who engage in high-risk behaviors – IV or intranasal drug abuse – unprotected sex with an infected partner or > 1 partner – Hx of STD • All internationally adopted children • Immigrants from high-prevalence areas – Africa, SE Asia, the Middle East except Israel, the interior Amazon River basin, Haiti/D. R. • Children living in communities where HBV is endemic • Household contacts of individuals with HBV infection • Infants born to women with HBV infection – If infant got hepatitis B immune globulin and hepatitis B vaccine at birth, followed by two additional immunizations, test at 9 -15 m – Unimmunized should be tested as soon as identified

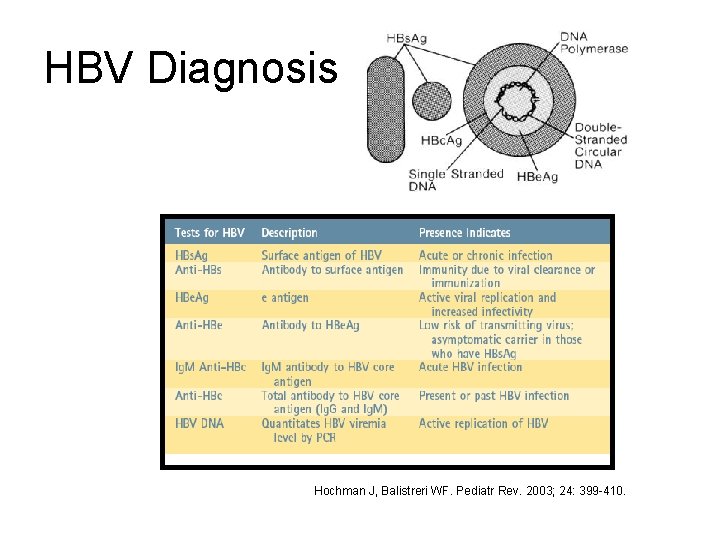
HBV Diagnosis Hochman J, Balistreri WF. Pediatr Rev. 2003; 24: 399 -410.

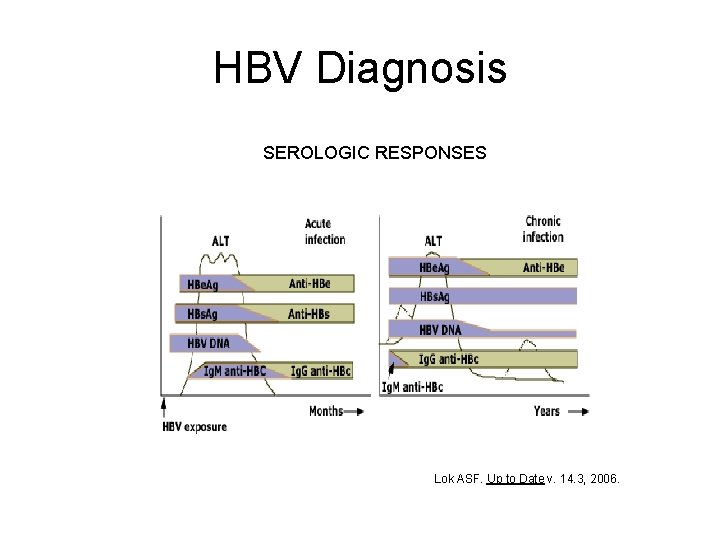
HBV Diagnosis SEROLOGIC RESPONSES Lok ASF. Up to Date v. 14. 3, 2006.

HBV Serology • • HBs. Ag: + Anti-HBc: + Ig. M anti-HBc: + Anti-HBs: INTERPRETATION: Acute HBV infection • • HBs. Ag: + Anti-HBc: + Ig. M anti-HBc: Anti-HBs: INTERPRETATION: Chronic HBV infection

HBV Serology • HBs. Ag: • Anti-HBc: + • Anti-HBs: + INTERPRETATION: Immunity due to natural infection • HBs. Ag: • Anti-HBc: • Anti-HBs: + INTERPRETATION: Immunity due to HBV vaccination

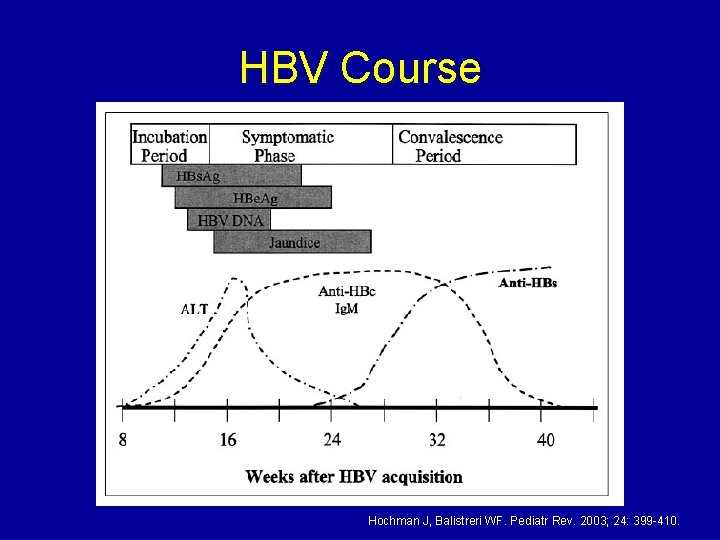
HBV Course Hochman J, Balistreri WF. Pediatr Rev. 2003; 24: 399 -410.

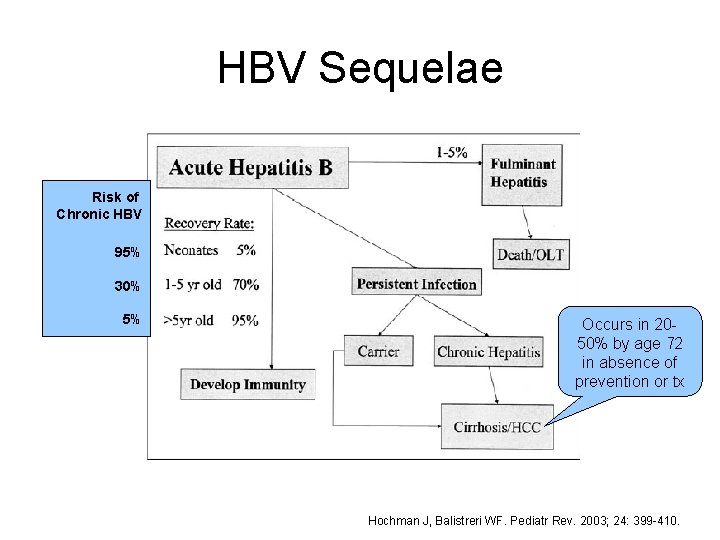
HBV Sequelae Risk of Chronic HBV 95% 30% 5% Occurs in 2050% by age 72 in absence of prevention or tx Hochman J, Balistreri WF. Pediatr Rev. 2003; 24: 399 -410.

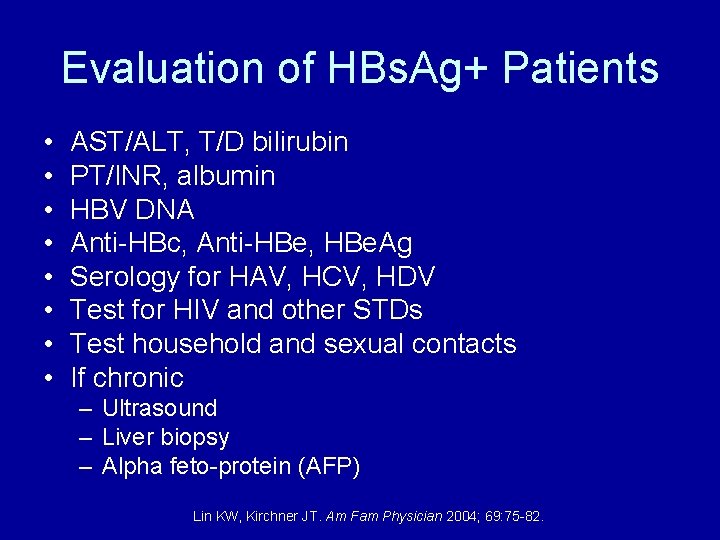
Evaluation of HBs. Ag+ Patients • • AST/ALT, T/D bilirubin PT/INR, albumin HBV DNA Anti-HBc, Anti-HBe, HBe. Ag Serology for HAV, HCV, HDV Test for HIV and other STDs Test household and sexual contacts If chronic – Ultrasound – Liver biopsy – Alpha feto-protein (AFP) Lin KW, Kirchner JT. Am Fam Physician 2004; 69: 75 -82.

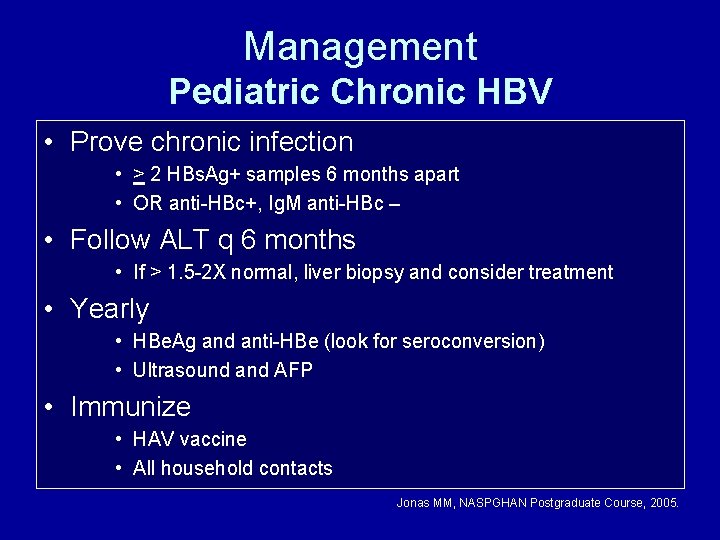
Management Pediatric Chronic HBV • Prove chronic infection • > 2 HBs. Ag+ samples 6 months apart • OR anti-HBc+, Ig. M anti-HBc – • Follow ALT q 6 months • If > 1. 5 -2 X normal, liver biopsy and consider treatment • Yearly • HBe. Ag and anti-HBe (look for seroconversion) • Ultrasound and AFP • Immunize • HAV vaccine • All household contacts Jonas MM, NASPGHAN Postgraduate Course, 2005.

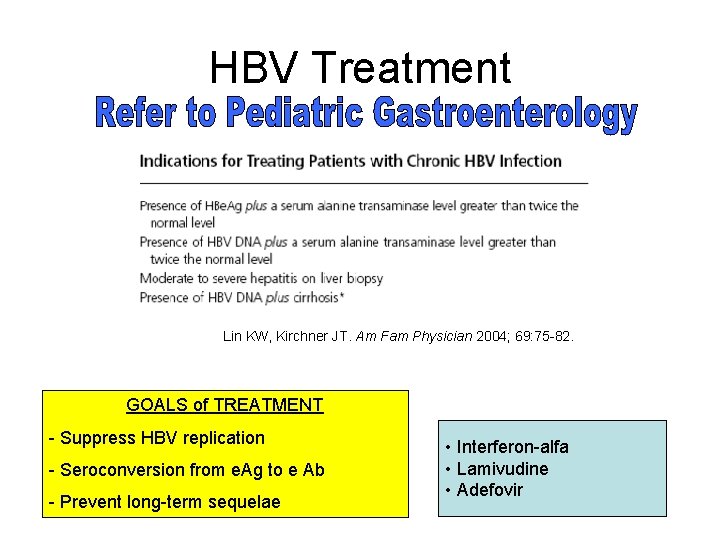
HBV Treatment Lin KW, Kirchner JT. Am Fam Physician 2004; 69: 75 -82. GOALS of TREATMENT - Suppress HBV replication - Seroconversion from e. Ag to e Ab - Prevent long-term sequelae • Interferon-alfa • Lamivudine • Adefovir

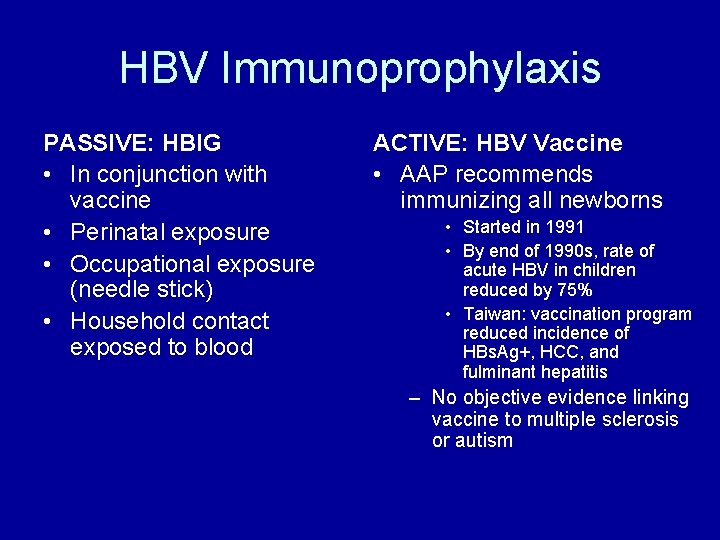
HBV Immunoprophylaxis PASSIVE: HBIG • In conjunction with vaccine • Perinatal exposure • Occupational exposure (needle stick) • Household contact exposed to blood ACTIVE: HBV Vaccine • AAP recommends immunizing all newborns • Started in 1991 • By end of 1990 s, rate of acute HBV in children reduced by 75% • Taiwan: vaccination program reduced incidence of HBs. Ag+, HCC, and fulminant hepatitis – No objective evidence linking vaccine to multiple sclerosis or autism

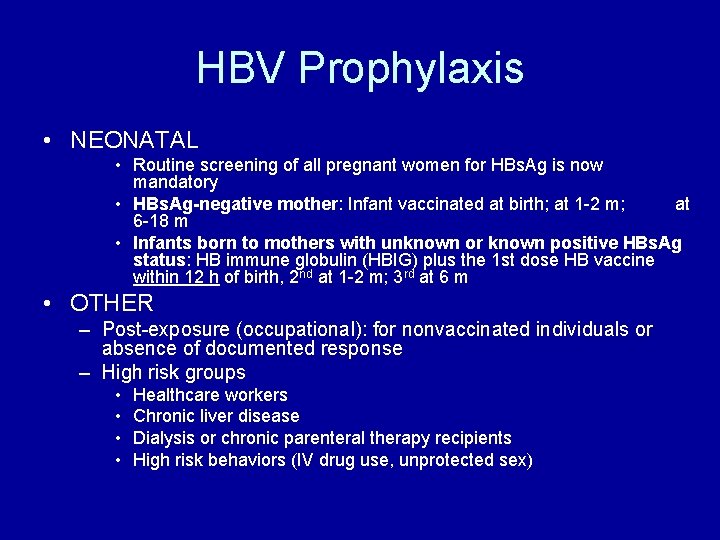
HBV Prophylaxis • NEONATAL • Routine screening of all pregnant women for HBs. Ag is now mandatory • HBs. Ag-negative mother: Infant vaccinated at birth; at 1 -2 m; at 6 -18 m • Infants born to mothers with unknown or known positive HBs. Ag status: HB immune globulin (HBIG) plus the 1 st dose HB vaccine within 12 h of birth, 2 nd at 1 -2 m; 3 rd at 6 m • OTHER – Post-exposure (occupational): for nonvaccinated individuals or absence of documented response – High risk groups • • Healthcare workers Chronic liver disease Dialysis or chronic parenteral therapy recipients High risk behaviors (IV drug use, unprotected sex)

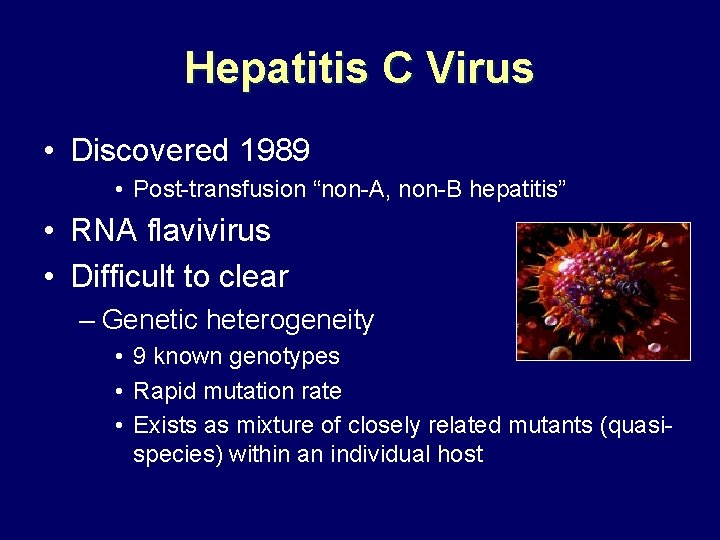
Hepatitis C Virus • Discovered 1989 • Post-transfusion “non-A, non-B hepatitis” • RNA flavivirus • Difficult to clear – Genetic heterogeneity • 9 known genotypes • Rapid mutation rate • Exists as mixture of closely related mutants (quasispecies) within an individual host

HCV Epidemiology • Prevalence • Adults: 2% • Children: 0. 2%, Adolescents: 0. 4% • Transmission – Maternal-fetal • Mother HCV+: 5% risk • Mother co-infected with HIV: 15% risk • All infants born to HCV-infected mothers should be tested for anti-HCV Ab after 12 m of age – Blood transfusion – Screening blood products has reduced risk to <1: 100, 000 – Other risk factors – High risk sexual behavior – IV drug abuse – Tattooing, body piercing

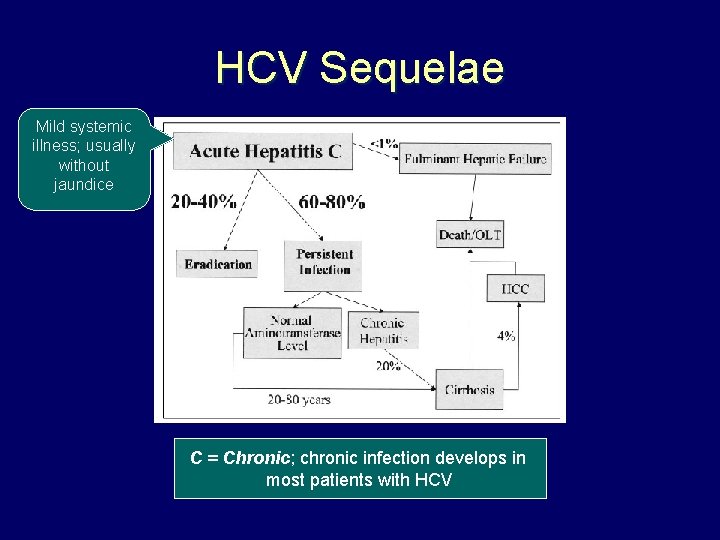
HCV Sequelae Mild systemic illness; usually without jaundice C = Chronic; chronic infection develops in most patients with HCV

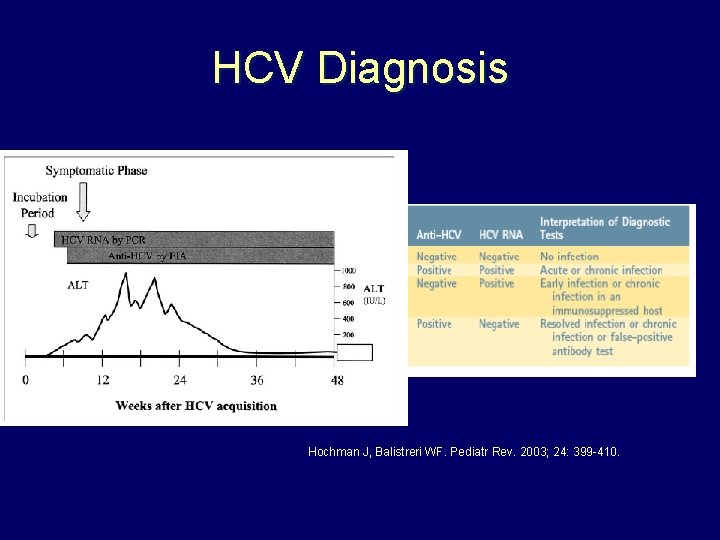
HCV Diagnosis Hochman J, Balistreri WF. Pediatr Rev. 2003; 24: 399 -410.

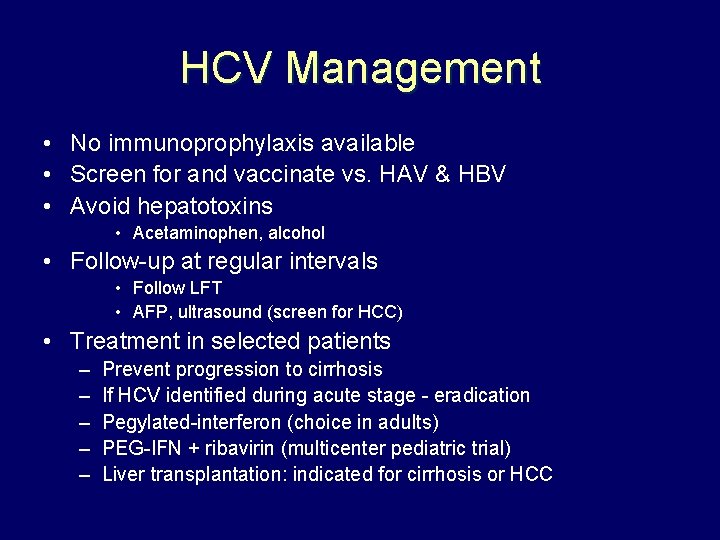
HCV Management • No immunoprophylaxis available • Screen for and vaccinate vs. HAV & HBV • Avoid hepatotoxins • Acetaminophen, alcohol • Follow-up at regular intervals • Follow LFT • AFP, ultrasound (screen for HCC) • Treatment in selected patients – – – Prevent progression to cirrhosis If HCV identified during acute stage - eradication Pegylated-interferon (choice in adults) PEG-IFN + ribavirin (multicenter pediatric trial) Liver transplantation: indicated for cirrhosis or HCC

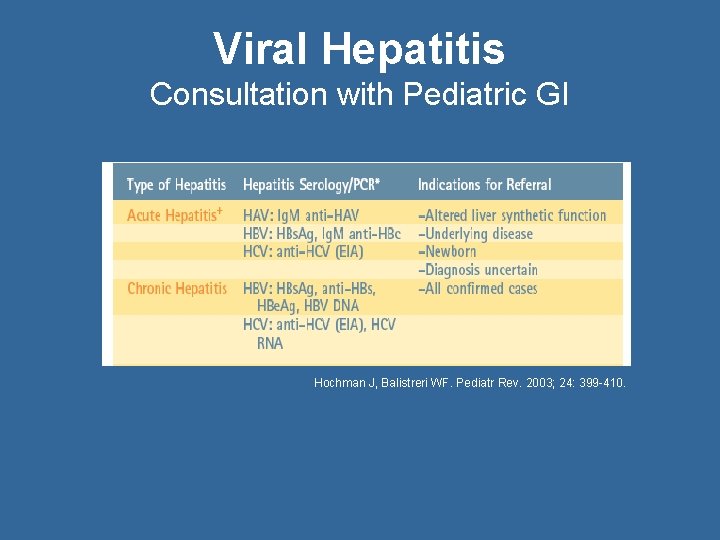
Viral Hepatitis Consultation with Pediatric GI Hochman J, Balistreri WF. Pediatr Rev. 2003; 24: 399 -410.

Breastfeeding • HBs. Ag+ mothers – can breastfeed as long as neonate has received HBIG and vaccine within 12 h of birth • HCV+ mother is not contraindication to breastfeeding – Data re: transmission in human milk is limited

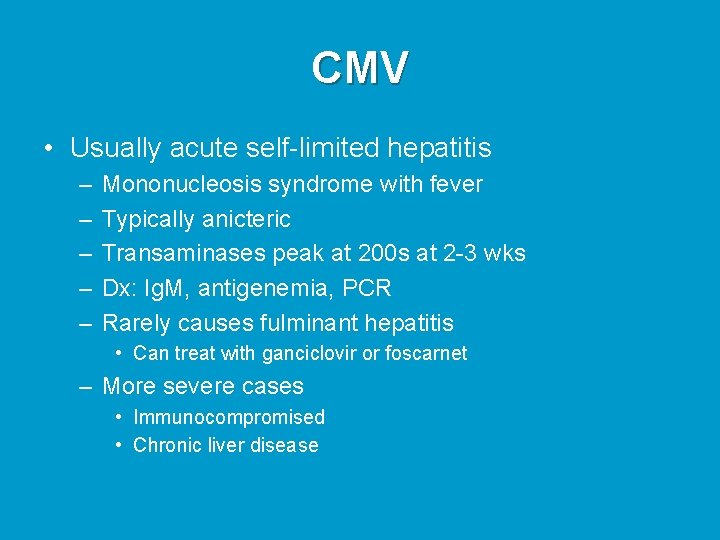
CMV • Usually acute self-limited hepatitis – – – Mononucleosis syndrome with fever Typically anicteric Transaminases peak at 200 s at 2 -3 wks Dx: Ig. M, antigenemia, PCR Rarely causes fulminant hepatitis • Can treat with ganciclovir or foscarnet – More severe cases • Immunocompromised • Chronic liver disease

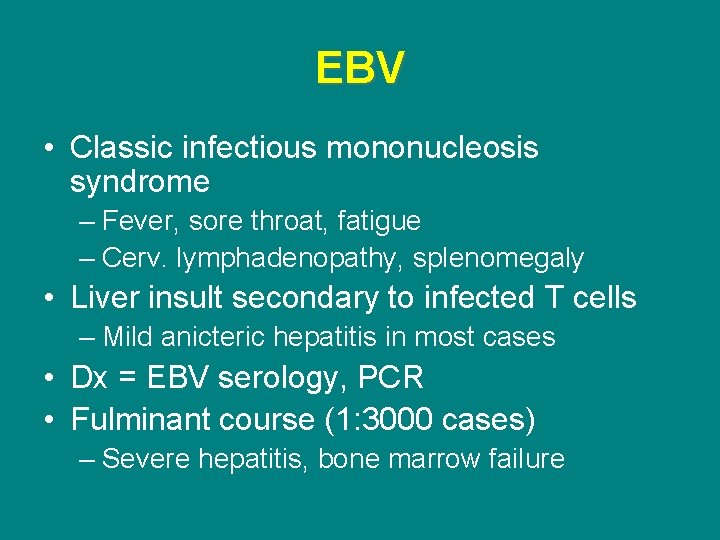
EBV • Classic infectious mononucleosis syndrome – Fever, sore throat, fatigue – Cerv. lymphadenopathy, splenomegaly • Liver insult secondary to infected T cells – Mild anicteric hepatitis in most cases • Dx = EBV serology, PCR • Fulminant course (1: 3000 cases) – Severe hepatitis, bone marrow failure

North American Society for Pediatric Gastroenterology, Hepatology and Nutrition www. naspghan. org www. cdhnf. org www. hepb. org www. aasld. org
 Hgado
Hgado Gains in perspective taking permit the transition to
Gains in perspective taking permit the transition to Infants and children 8th edition
Infants and children 8th edition Lara berk
Lara berk Infants and children 8th edition
Infants and children 8th edition Section 24-1 viral structure and replication
Section 24-1 viral structure and replication Hepatitis symptoms
Hepatitis symptoms Viral induced wheeze vs asthma
Viral induced wheeze vs asthma Replicação viral ciclo lítico e lisogênico
Replicação viral ciclo lítico e lisogênico Inklüzyon cisimcikleri
Inklüzyon cisimcikleri Method of cultivation of virus
Method of cultivation of virus Viral inoculation in embryonated egg
Viral inoculation in embryonated egg Viral inoculation in embryonated egg
Viral inoculation in embryonated egg Spasmodic croup
Spasmodic croup Dea anggraini viral
Dea anggraini viral Decapsidação
Decapsidação Hemolyzed serum sample
Hemolyzed serum sample Que causa la meningitis
Que causa la meningitis Causes of viral hemorrhagic fever
Causes of viral hemorrhagic fever Inmunidad
Inmunidad Viral infection
Viral infection Viral
Viral Viral recombination
Viral recombination Vaccins à vecteur viral
Vaccins à vecteur viral Viral receptors
Viral receptors Viral
Viral Rhinopneumonitis definition
Rhinopneumonitis definition Virus dna
Virus dna Dha mcq
Dha mcq Viral entry
Viral entry The dynamics of viral marketing
The dynamics of viral marketing Csf analysis interpretation
Csf analysis interpretation Viral arthritis
Viral arthritis An acute highly contagious viral disease
An acute highly contagious viral disease Viral life cycle
Viral life cycle Antiperytique
Antiperytique Viral shedding
Viral shedding Eline's viral
Eline's viral Viral communications
Viral communications Viral integration
Viral integration Trplice
Trplice