Vibrio spp Vibrio parahaemolyticus vibriothe shape of bacteria
Vibrio spp. 弧菌屬

• • • 腸炎弧菌(Vibrio parahaemolyticus) vibrio-the shape of bacteria para-副、minor haemo-blood lyticus-lysis 副溶血弧菌 (中國譯名) Non-spore forming, Gram negative bacteria, polar monochromic flagella, salt needed, generation time: 10 -12 min at suitable environment. • Distribution and contamination route • Sea water or river delta, thus, seafood is the major carrier of this bacterium. It is common to occur in other foods through crosscontamination.

• Associated foods and symptom • The main cause is seafood or other foods cross-contaminated by seafood. Incubation time is 2 -48 hours and average incubation time is 10 -18 hours. The shorter of incubation time, the severe of symptom. The main symptoms are watery diarrhea, abdominal pains and/or cramping, nausea, vomiting, fever and chills. • prevention • 1. washing: V. parahaemolyticus cannot grow in fresh water. Washing foods with enough amount of fresh water can reduce the bacterial populations. 2. heating: 60℃ for l 5 minutes can kill this bacterium. 3. refrigerate or freeze: V. parahaemolyticus is sensitive to cold, store below 1 O℃ can inhibit the growth, freezing can kill this bacterium. 4. Do not eat raw seafood, no sashimi. 5. prevent cross-contamination


Vibrio parahaemolyticus • The first case occurred in Japan 1950 272 patients, 20 death • At beginning, it was named as Pasteurella haemolyticus, changed to current name in 1963. • Sea associated, higher populations in summer, live in sediment in winter.

Vibrio parahaemolyticus • The most occurring pathogen in Japan, Korea, and Taiwan. • Virulent strains of V. parahaemolyticus have Kanagawa phenomenon (KP+) which means the strains show betahemolysis on Wagatsuma agar. This phenomenon is caused by the Thermostable direct hemolysin (TDH).

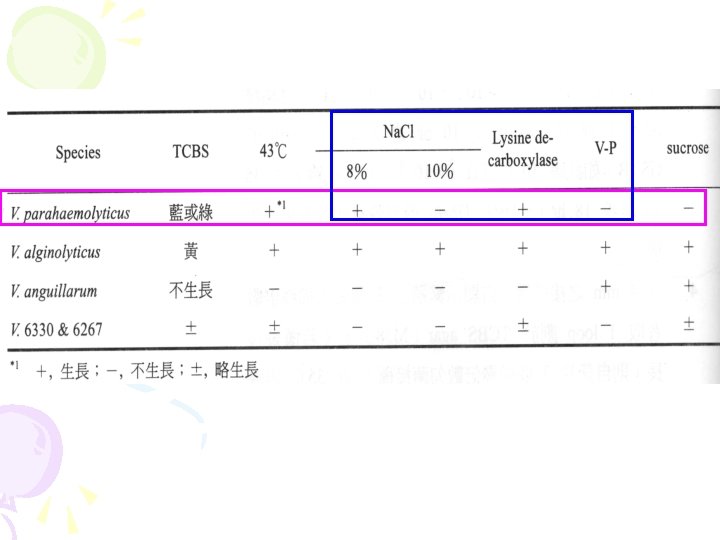
Vibrio parahaemolyticus • TDH is heat stable and toxicity is weaker than chlorae toxin which is produced by V. cholerae. • Besides TDH, there are some minor toxins, such as lipooplysaccharides (LPS). • Very few KP- strains also can induce disease. • Serotypes of V. parahaemolyticus is depended on O (cell surface) and K (capsule) antigens. The most important serotype is O 3: K 6.
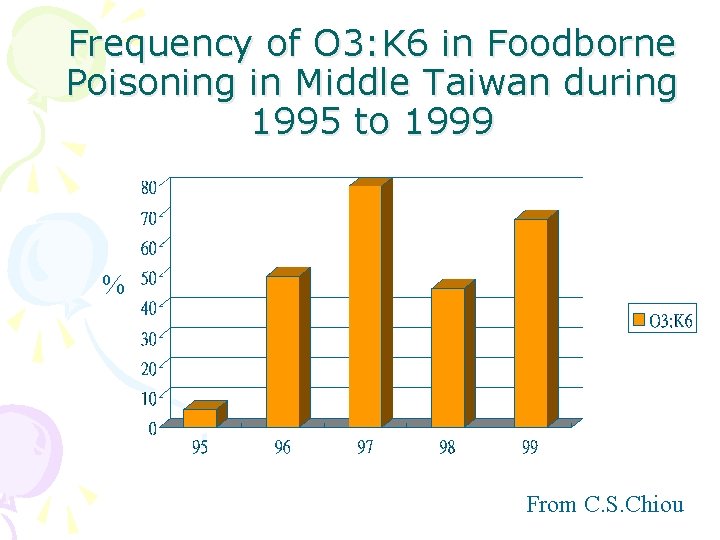
Frequency of O 3: K 6 in Foodborne Poisoning in Middle Taiwan during 1995 to 1999 % From C. S. Chiou

Other vibrios • V. cholerae • V. vulnificus 創傷弧菌 • V. mimicus • V. fluvialis • V. furnissii • V. hollisae • V. damsela
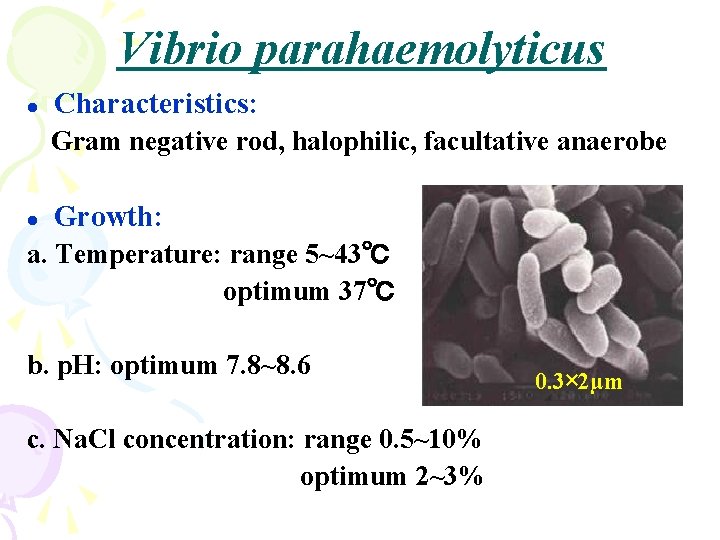
Vibrio parahaemolyticus l Characteristics: Gram negative rod, halophilic, facultative anaerobe l Growth: a. Temperature: range 5~43℃ optimum 37℃ b. p. H: optimum 7. 8~8. 6 c. Na. Cl concentration: range 0. 5~10% optimum 2~3% 0. 3× 2µm
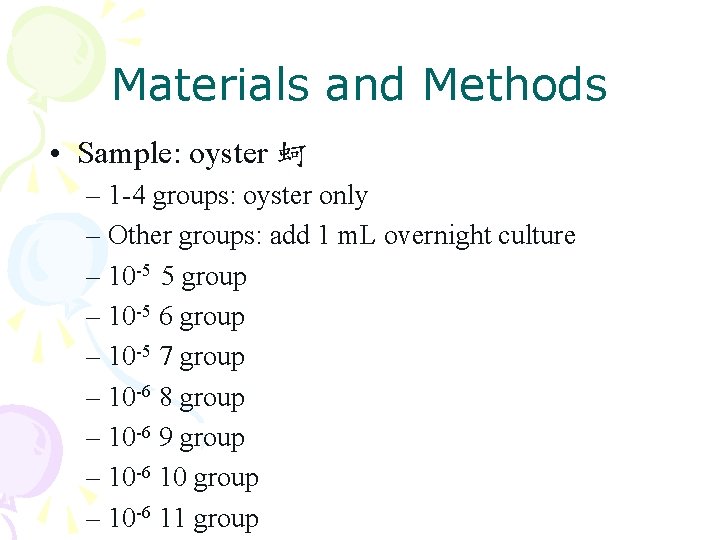
Materials and Methods • Sample: oyster 蚵 – 1 -4 groups: oyster only – Other groups: add 1 m. L overnight culture – 10 -5 5 group – 10 -5 6 group – 10 -5 7 group – 10 -6 8 group – 10 -6 9 group – 10 -6 10 group – 10 -6 11 group
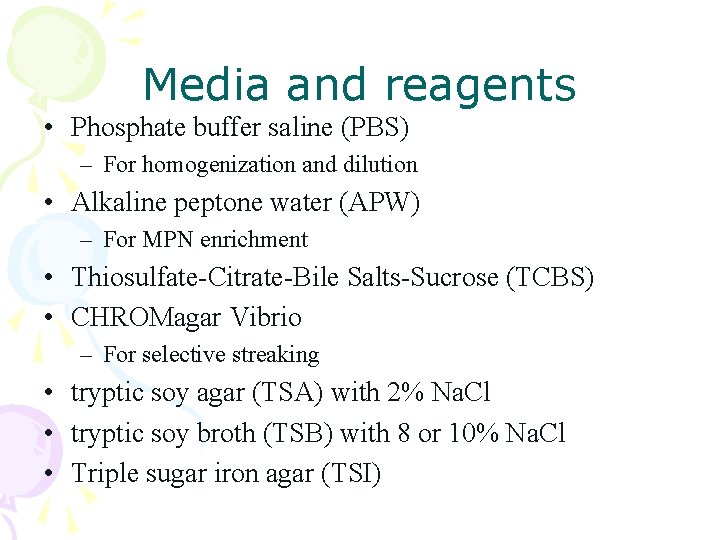
Media and reagents • Phosphate buffer saline (PBS) – For homogenization and dilution • Alkaline peptone water (APW) – For MPN enrichment • Thiosulfate-Citrate-Bile Salts-Sucrose (TCBS) • CHROMagar Vibrio – For selective streaking • tryptic soy agar (TSA) with 2% Na. Cl • tryptic soy broth (TSB) with 8 or 10% Na. Cl • Triple sugar iron agar (TSI)
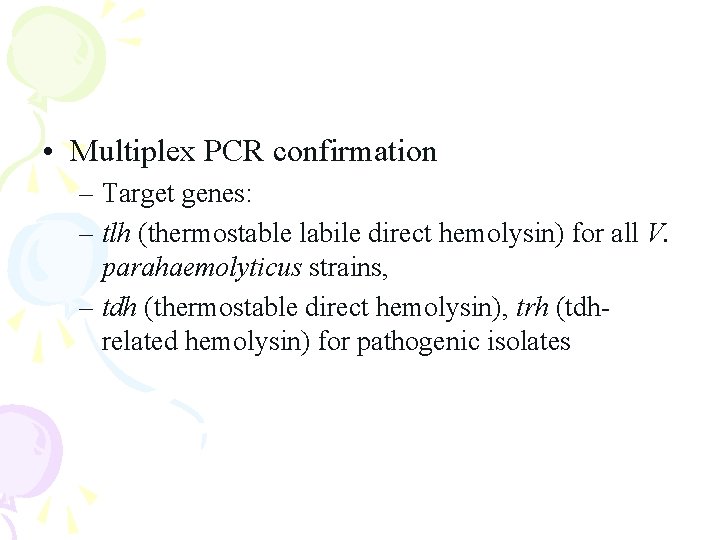
• Multiplex PCR confirmation – Target genes: – tlh (thermostable labile direct hemolysin) for all V. parahaemolyticus strains, – tdh (thermostable direct hemolysin), trh (tdhrelated hemolysin) for pathogenic isolates
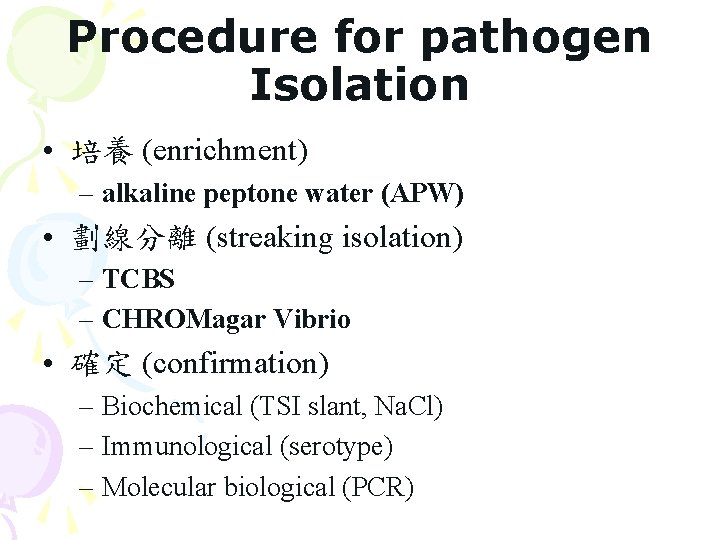
Procedure for pathogen Isolation • 培養 (enrichment) – alkaline peptone water (APW) • 劃線分離 (streaking isolation) – TCBS – CHROMagar Vibrio • 確定 (confirmation) – Biochemical (TSI slant, Na. Cl) – Immunological (serotype) – Molecular biological (PCR)
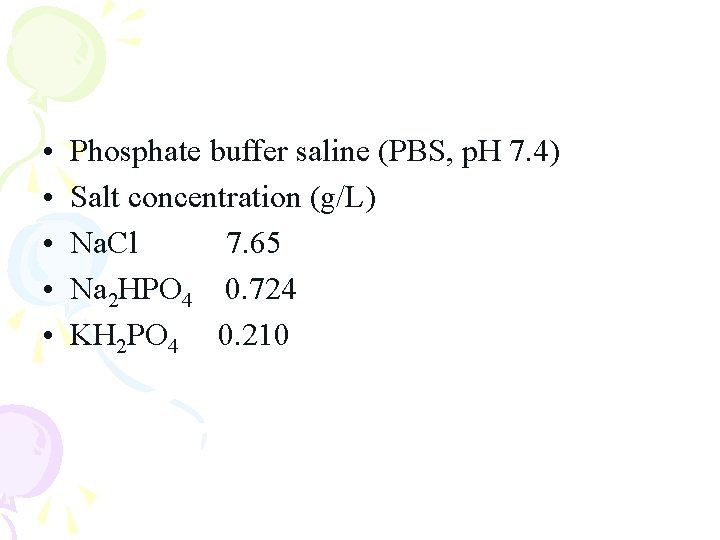
• • • Phosphate buffer saline (PBS, p. H 7. 4) Salt concentration (g/L) Na. Cl 7. 65 Na 2 HPO 4 0. 724 KH 2 PO 4 0. 210
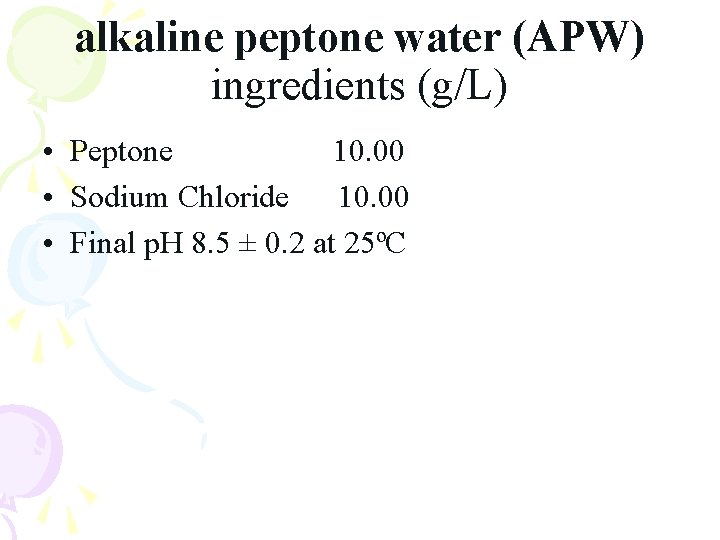
alkaline peptone water (APW) ingredients (g/L) • Peptone 10. 00 • Sodium Chloride 10. 00 • Final p. H 8. 5 ± 0. 2 at 25ºC
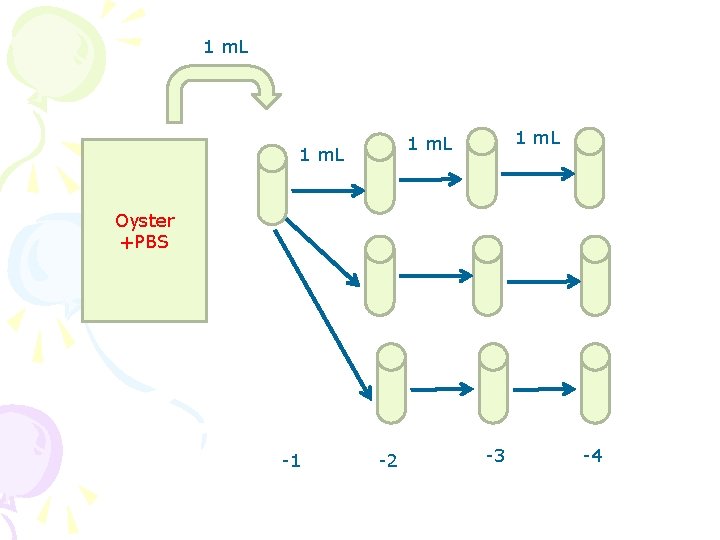
1 m. L Oyster +PBS -1 -2 -3 -4

• Sample preparation – Place 25 g of oyster sample into a stomach bag containing 225 m. L PBS – Stomach or homogenize for one minute (one sample for two groups) • 1 -4 groups: – Transfer one m. L of the homogenate into a tube containing 9 m. L APW – transfer into three tube (one m. L for each tube) – Serial transfer into further 6 tubes (total 9 tubes) – Final dilution, -2, -3 -4

• 5 -8 groups: • Add one m. L of overnight culture into the homogenate • Transfer one m. L of the homogenate into a tube containing 9 m. L APW • Final dilution, -2, -3, -4 • 9 -11 groups: – transfer into three tube (one m. L for each tube) – Serial transfer into further 6 tubes (total 9 tubes) – Final dilution, -3, -4, -5
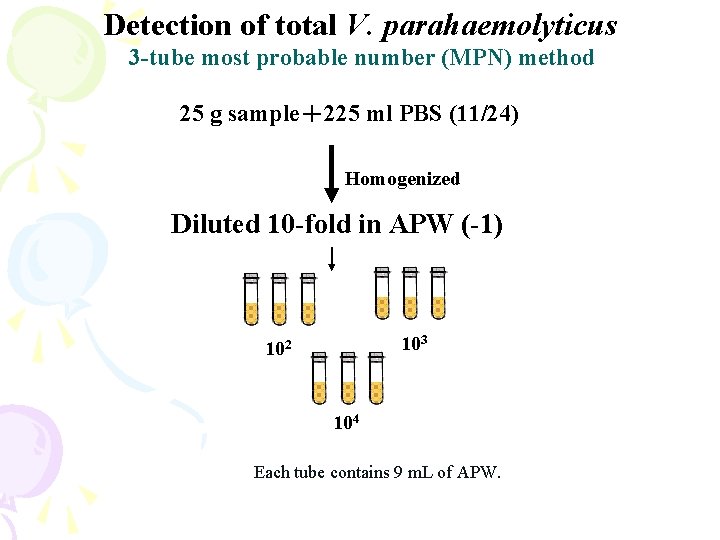
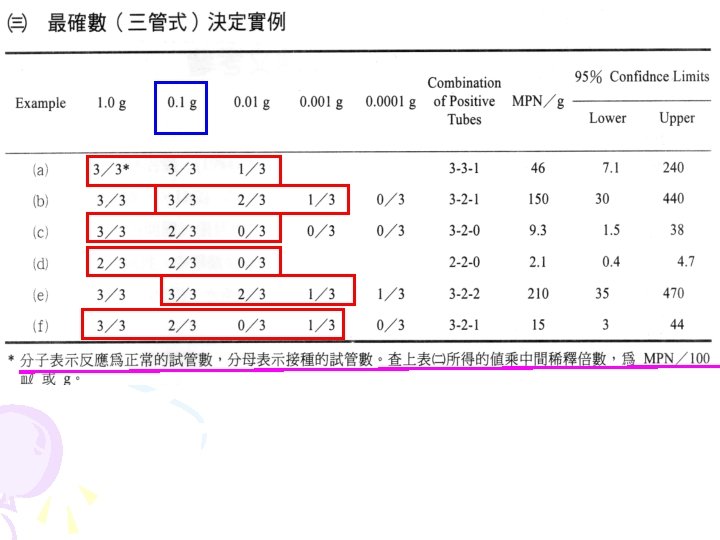
Detection of total V. parahaemolyticus 3 -tube most probable number (MPN) method 25 g sample+225 ml PBS (11/24) Homogenized Diluted 10 -fold in APW (-1) 103 102 104 Each tube contains 9 m. L of APW.
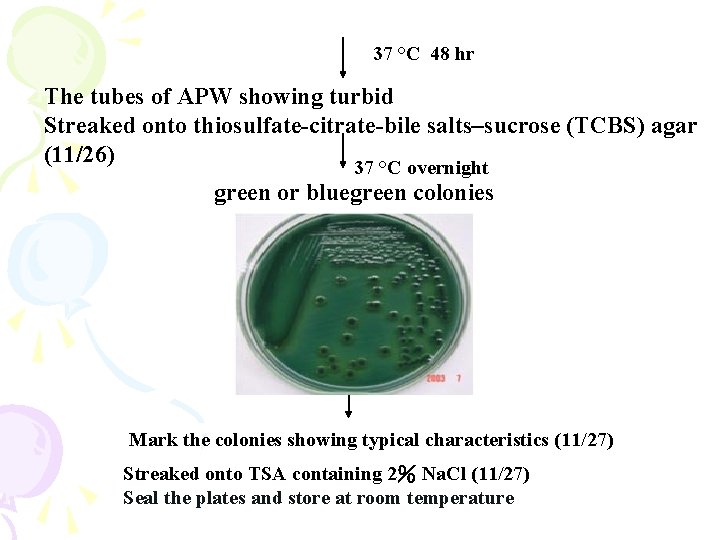
37 °C 48 hr The tubes of APW showing turbid Streaked onto thiosulfate-citrate-bile salts–sucrose (TCBS) agar (11/26) 37 °C overnight green or bluegreen colonies Mark the colonies showing typical characteristics (11/27) Streaked onto TSA containing 2% Na. Cl (11/27) Seal the plates and store at room temperature
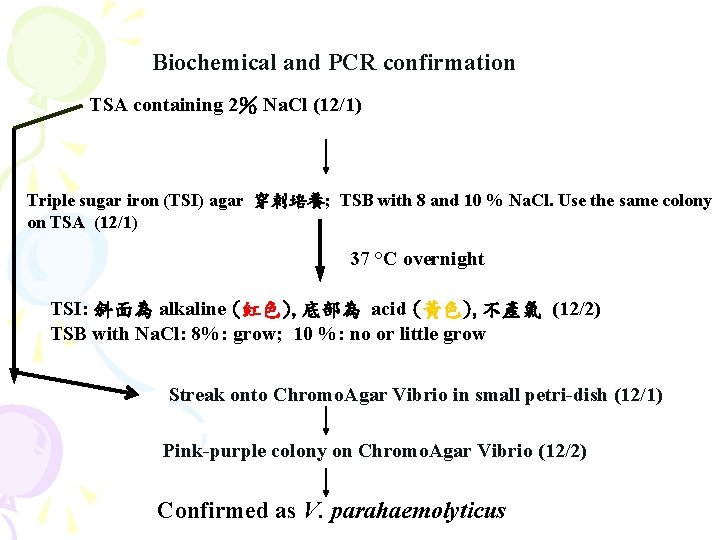
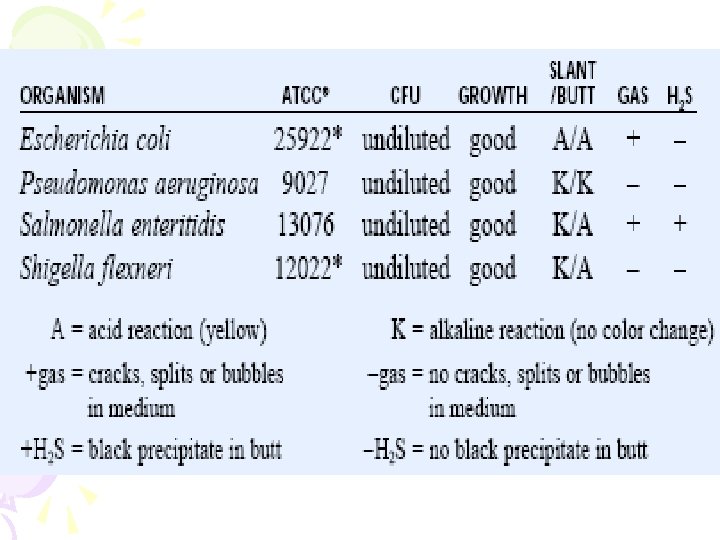
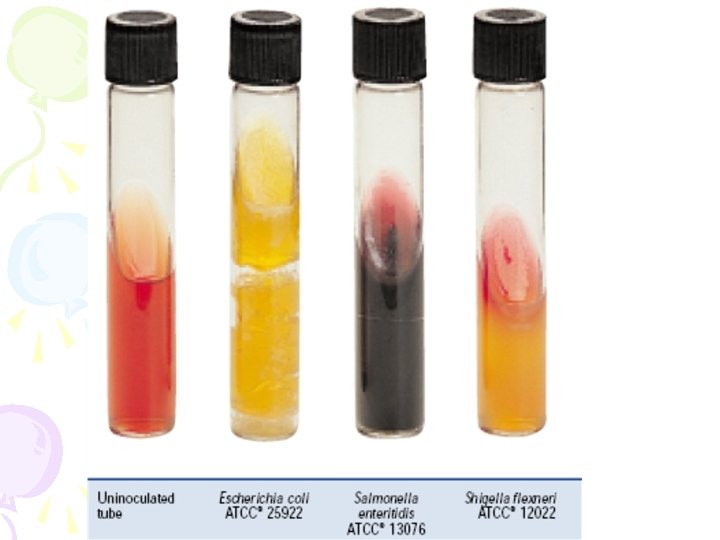
Biochemical and PCR confirmation TSA containing 2% Na. Cl (12/1) Triple sugar iron (TSI) agar 穿刺培養; TSB with 8 and 10 % Na. Cl. Use the same colony on TSA (12/1) 37 °C overnight TSI: 斜面為 alkaline (紅色), 底部為 acid (黃色), 不產氣 (12/2) TSB with Na. Cl: 8%: grow; 10 %: no or little grow Streak onto Chromo. Agar Vibrio in small petri-dish (12/1) Pink-purple colony on Chromo. Agar Vibrio (12/2) Confirmed as V. parahaemolyticus

Selective medium TCBS V. parahaemolyticus – green V. vulnificus - green Chromoagar V. parahaemolyticus – pink V. vulnificus - blue

Schedule • 11/24 (Mon) – Vibrio parahaemolyticus sample preparation and MPN • 11/26(Wed) – 劃 TCBS plates,分離V. parahaemolyticus • 11/27 (Thu) – 標定典型菌落 – 劃在TSA- 2% Na. Cl plate 上 – 放室溫下保存 • 12/1 (Mon) – – 接種 TSI, Streak onto Chromo. Agar Na. Cl growth 鹽生長試驗 (TSB with 8%, 10% Na. Cl) PCR – 12/2 (Tue) 觀察結果
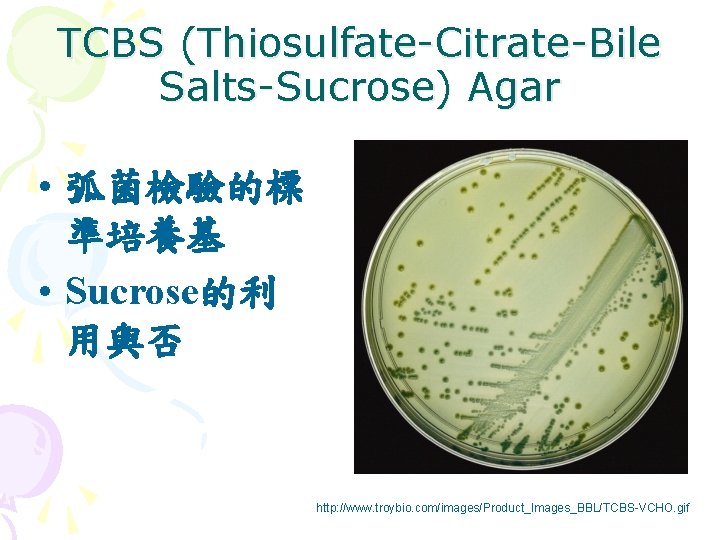
TCBS (Thiosulfate-Citrate-Bile Salts-Sucrose) Agar • 弧菌檢驗的標 準培養基 • Sucrose的利 用與否 http: //www. troybio. com/images/Product_Images_BBL/TCBS-VCHO. gif

硫代硫酸鹽-檸檬酸鹽-膽鹽-蔗糖 培養基 (Thiosulfate-Citrate-Bile salts-Sucrose Agar) • • • Mixed peptone Yeast extract As a fermentable carbohydrate Sucrose Sodium citrate (檸檬酸鈉) Ferric citrate (檸檬酸鐵) Sodium chloride Inhibit G(+) Sodium thiosulfate (硫代硫酸鈉) Oxbile (牛膽汁) Sodium cholate (膽酸鈉) Thymol blue Changes its color to yellow, when acid is Bromothymol blue formed Agar Final p. H 8. 6
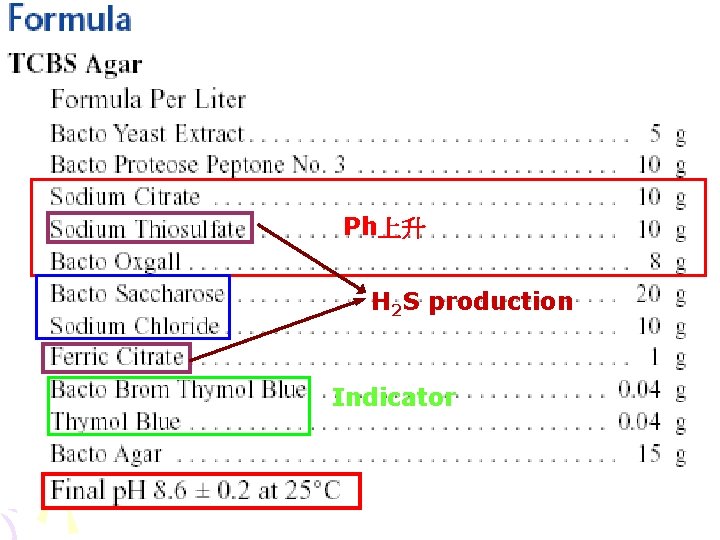
Ph上升 H 2 S production Indicator
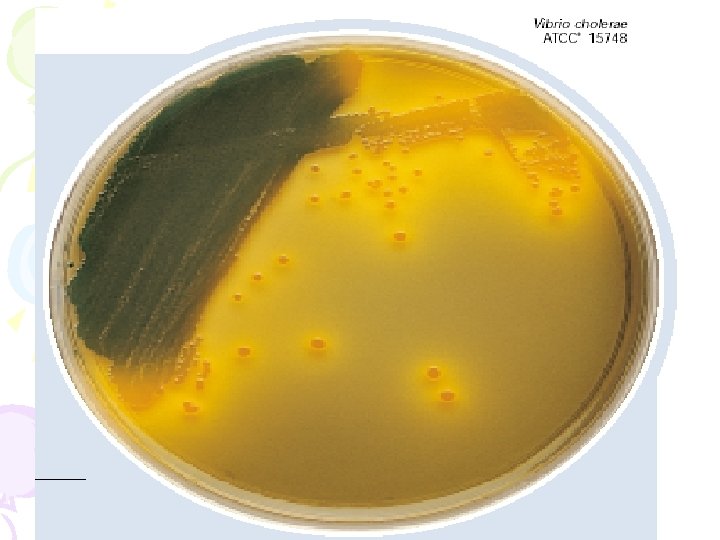
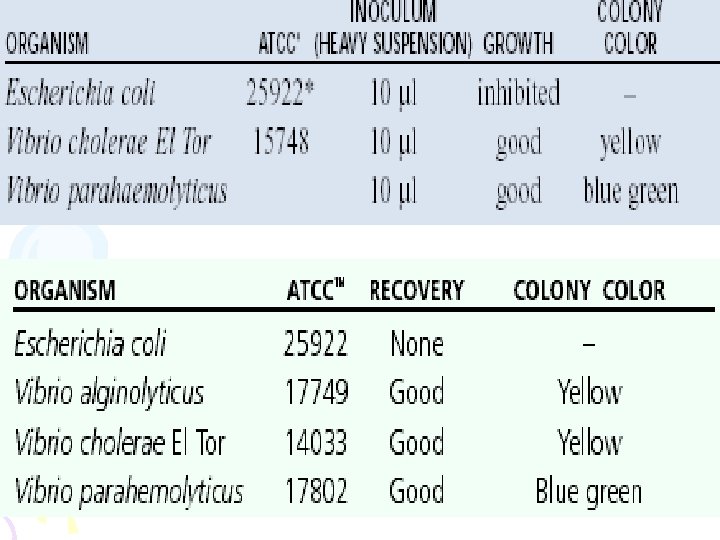

• CHROMagar Vibrio is a chromogenic media, for isolation of specimens, giving detection of Vibrio parahaemolyticus by colony color. • CHROMagar Vibrio can be used with spread plate procedures: prepare CHROMagar Vibrio petri plates according to instructions, inoculate and incubate at 37ºC for 24 hours. • Vibrio parahaemolyticus develop as easily distinguishable mauve coloured colonies, clearly visible under normal lighting conditions. Vibrio cholerae develops as turquoise blue colonies. Other bacterial species are inhibited or give colorless colonies. • Vibrio parahaemolyticus - Mauve • Vibrio cholerae - Turquoise-Blue • Vibrio alginolyticus - Colorless • Other bacterial colonies - inhibited or colourless
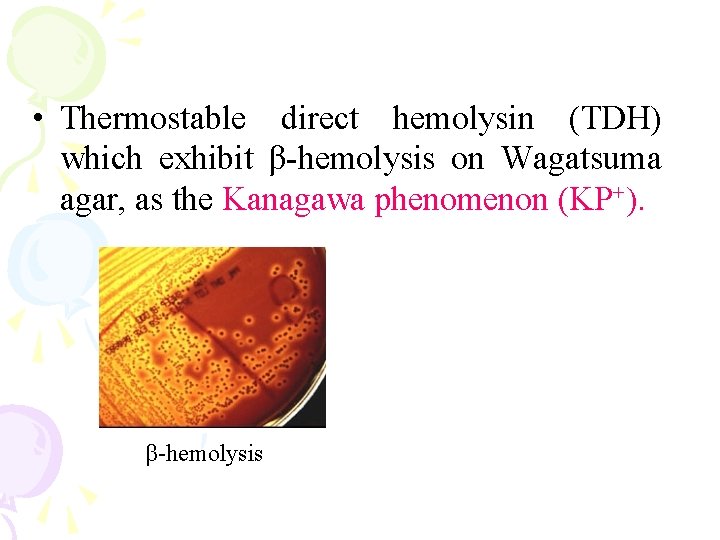
• Thermostable direct hemolysin (TDH) which exhibit β-hemolysis on Wagatsuma agar, as the Kanagawa phenomenon (KP+). β-hemolysis


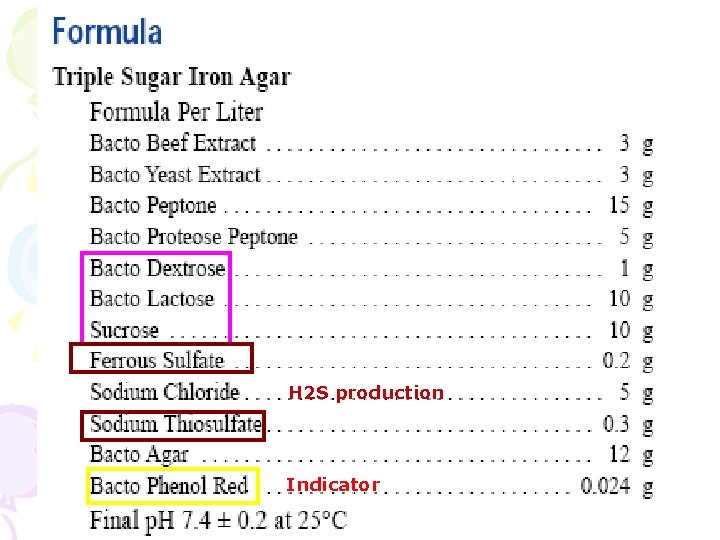
H 2 S production Indicator



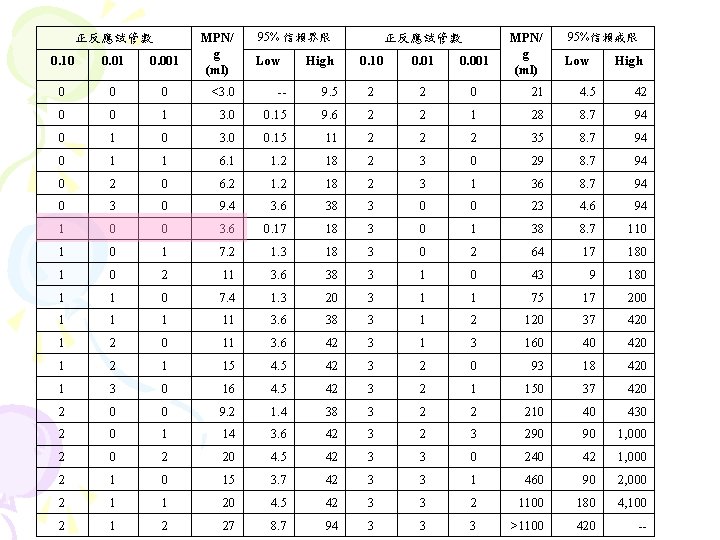
正反應試管數 MPN/ g (ml) 95% 信賴界限 0. 10 0. 01 0. 001 Low 0 0 0 <3. 0 -- 0 0 1 3. 0 0 1 0 High 正反應試管數 MPN/ g (ml) 95%信賴戒限 0. 10 0. 01 0. 001 Low High 9. 5 2 2 0 21 4. 5 42 0. 15 9. 6 2 2 1 28 8. 7 94 3. 0 0. 15 11 2 2 2 35 8. 7 94 1 6. 1 1. 2 18 2 3 0 29 8. 7 94 2 0 6. 2 18 2 3 1 36 8. 7 94 0 3 0 9. 4 3. 6 38 3 0 0 23 4. 6 94 1 0 0 3. 6 0. 17 18 3 0 1 38 8. 7 110 1 7. 2 1. 3 18 3 0 2 64 17 180 1 0 2 11 3. 6 38 3 1 0 43 9 180 1 1 0 7. 4 1. 3 20 3 1 1 75 17 200 1 11 3. 6 38 3 1 2 120 37 420 1 2 0 11 3. 6 42 3 160 40 420 1 2 1 15 4. 5 42 3 2 0 93 18 420 1 3 0 16 4. 5 42 3 2 1 150 37 420 2 0 0 9. 2 1. 4 38 3 2 2 210 40 430 2 0 1 14 3. 6 42 3 290 90 1, 000 2 20 4. 5 42 3 3 0 240 42 1, 000 2 1 0 15 3. 7 42 3 3 1 460 90 2, 000 2 1 1 20 4. 5 42 3 3 2 1100 180 4, 100 2 1 2 27 8. 7 94 3 3 3 >1100 420 --
- Slides: 44