Viabahn Long Stent Grafts for Reanalyzing Long SFA

Viabahn Long Stent Grafts for Reanalyzing Long SFA Lesions Synergism with DCB or Stand Alone Procedure? Thomas Zeller, MD University Heart Center Freiburg Bad Krozingen, Germany

Thomas Zeller, MD For the 12 months preceding this presentation, I disclose the following types of financial relationships: • • Honoraria received from: Abbott Vascular, Bard Peripheral Vascular, Veryan, Biotronik, Boston Scientific Corp. , Cook Medical, Gore & Associates, Medtronic, Philips-Spectranetics, Tri. Reme, Veryan, Shockwave, Biotronik Consulted for: Boston Scientific Corp. , Cook Medical, Gore & Associates, Medtronic, Spectranetics, Veryan, B. Braun Research, clinical trial, or drug study funds received from: Biotronik, 480 biomedical, Bard Peripheral Vascular, Veryan, Biotronik, Cook Medical, Gore & Associates, Abbott Vascular, Medtronic, Philips, Terumo, Tri. Reme, Veryan, Shockwave Common stock: Veryan, QT Medical
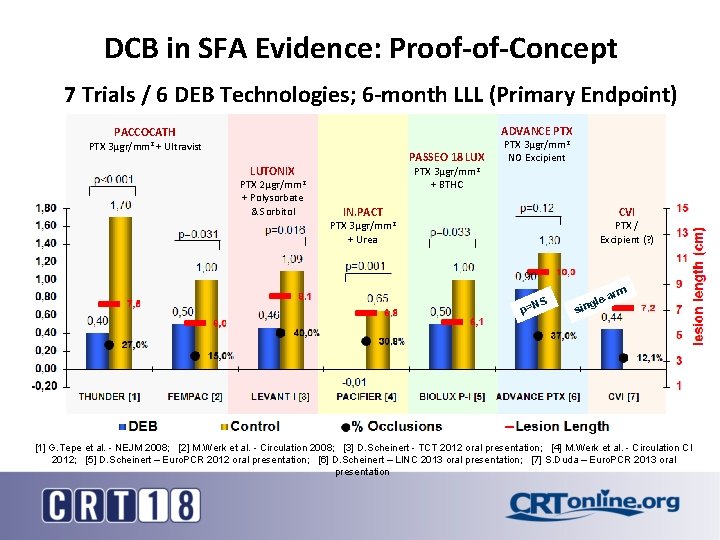
DCB in SFA Evidence: Proof-of-Concept 7 Trials / 6 DEB Technologies; 6 -month LLL (Primary Endpoint) PTX ADVANCE PTX PACCOCATH 3µgr/mm 2 + Ultravist PASSEO 18 LUX LUTONIX PTX 2µgr/mm 2 + Polysorbate & Sorbitol PTX 3µgr/mm 2 NO Excipient PTX 3µgr/mm 2 + BTHC IN. PACT PTX CVI 3µgr/mm 2 PTX / Excipient (? ) + Urea NS p= m -ar gle sin [1] G. Tepe et al. - NEJM 2008; [2] M. Werk et al. - Circulation 2008; [3] D. Scheinert - TCT 2012 oral presentation; [4] M. Werk et al. - Circulation CI 2012; [5] D. Scheinert – Euro. PCR 2012 oral presentation; [6] D. Scheinert – LINC 2013 oral presentation; [7] S. Duda – Euro. PCR 2013 oral presentation
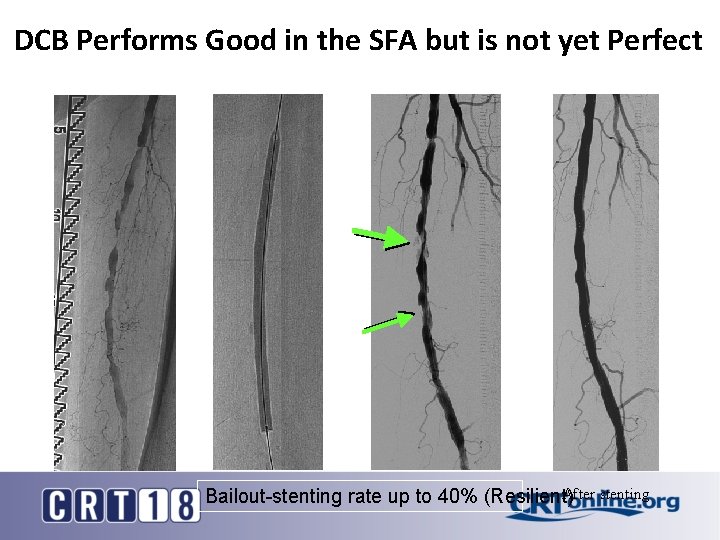
DCB Performs Good in the SFA but is not yet Perfect After stenting Bailout-stenting rate up to 40% (Resilient)
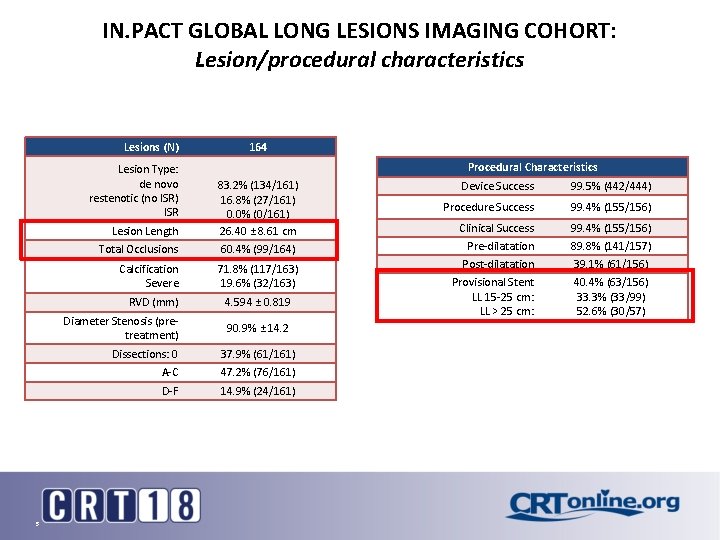
IN. PACT GLOBAL LONG LESIONS IMAGING COHORT: Lesion/procedural characteristics Lesions (N) Lesion Type: de novo restenotic (no ISR) ISR 5 164 Procedural Characteristics Lesion Length 83. 2% (134/161) 16. 8% (27/161) 0. 0% (0/161) 26. 40 ± 8. 61 cm Total Occlusions 60. 4% (99/164) Calcification Severe 71. 8% (117/163) 19. 6% (32/163) RVD (mm) 4. 594 ± 0. 819 Diameter Stenosis (pretreatment) 90. 9% ± 14. 2 Dissections: 0 37. 9% (61/161) A-C 47. 2% (76/161) D-F 14. 9% (24/161) Device Success 99. 5% (442/444) Procedure Success 99. 4% (155/156) Clinical Success Pre-dilatation Post-dilatation Provisional Stent LL 15 -25 cm: LL > 25 cm: 99. 4% (155/156) 89. 8% (141/157) 39. 1% (61/156) 40. 4% (63/156) 33. 3% (33/99) 52. 6% (30/57)
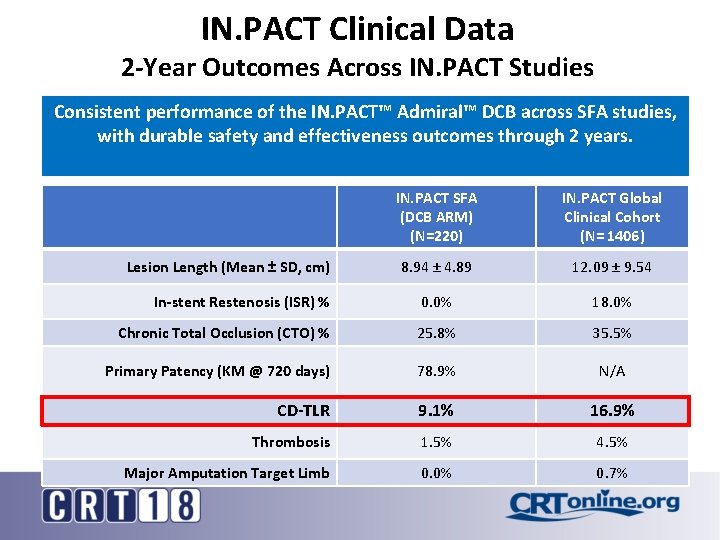
IN. PACT Clinical Data 2 -Year Outcomes Across IN. PACT Studies Consistent performance of the IN. PACT™ Admiral™ DCB across SFA studies, with durable safety and effectiveness outcomes through 2 years. IN. PACT SFA (DCB ARM) (N=220) IN. PACT Global Clinical Cohort (N= 1406) 8. 94 ± 4. 89 12. 09 ± 9. 54 In-stent Restenosis (ISR) % 0. 0% 18. 0% Chronic Total Occlusion (CTO) % 25. 8% 35. 5% Primary Patency (KM @ 720 days) 78. 9% N/A CD-TLR 9. 1% 16. 9% Thrombosis 1. 5% 4. 5% Major Amputation Target Limb 0. 0% 0. 7% Lesion Length (Mean ± SD, cm)
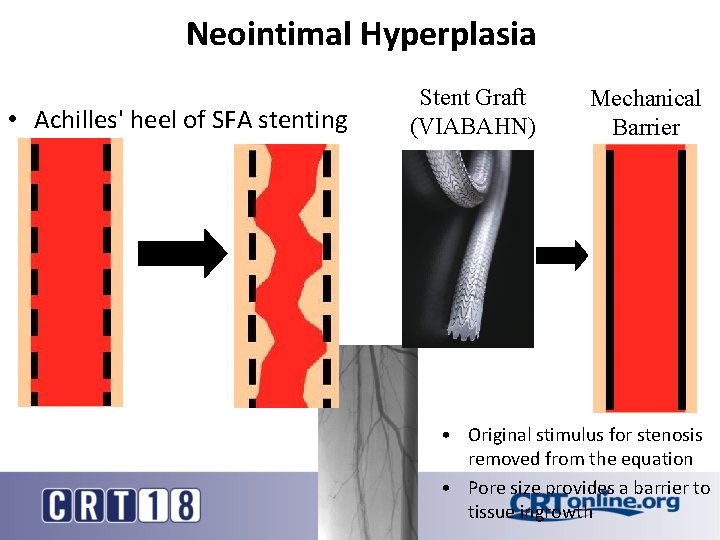
Neointimal Hyperplasia • Achilles' heel of SFA stenting Stent Graft (VIABAHN) Mechanical Barrier • Original stimulus for stenosis removed from the equation • Pore size provides a barrier to tissue ingrowth
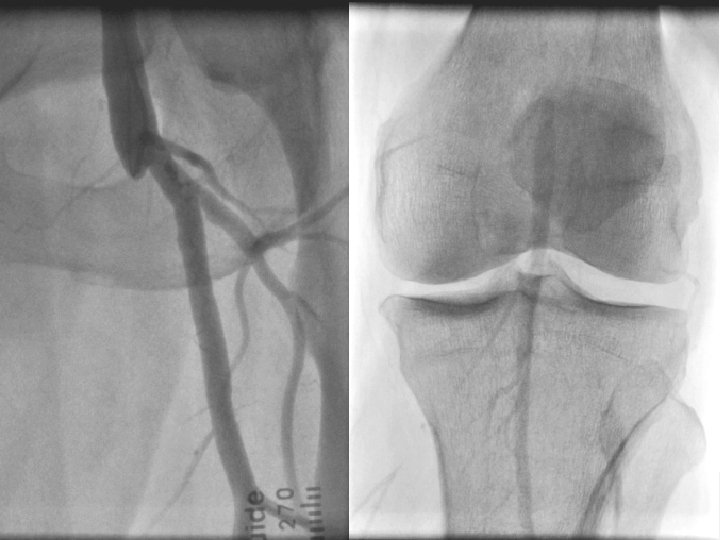
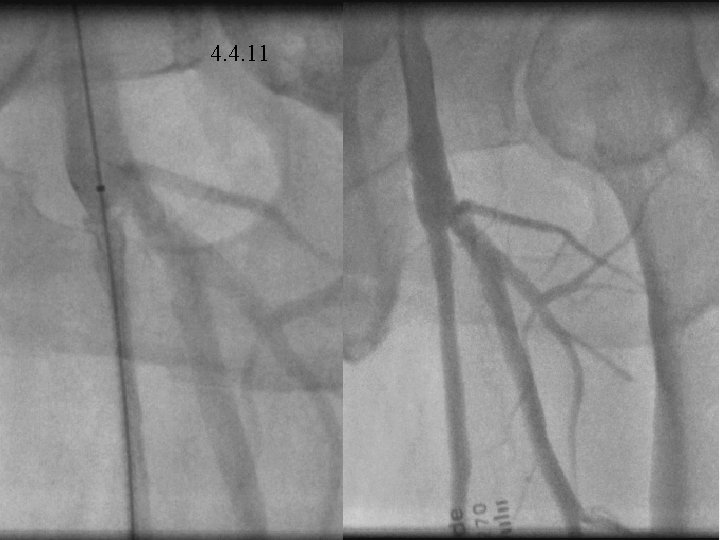
4. 4. 11
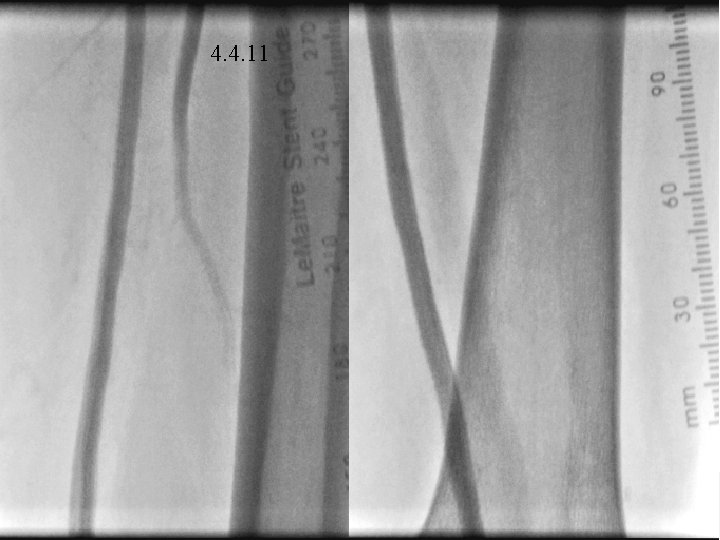
4. 4. 11
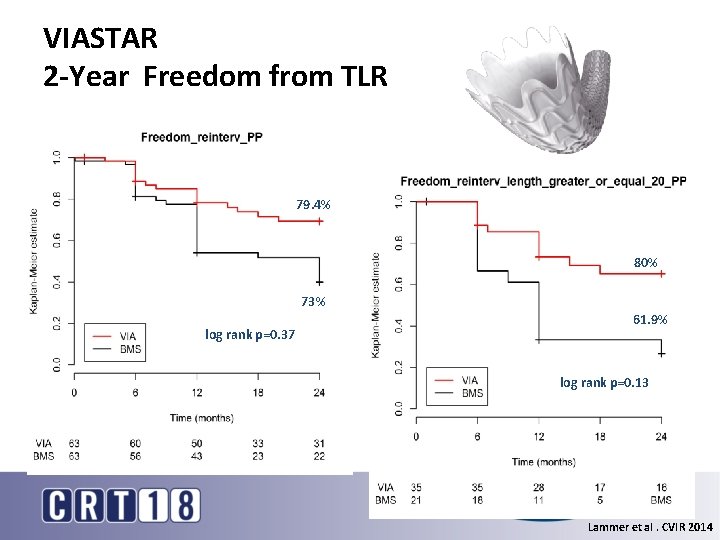
VIASTAR 2 -Year Freedom from TLR 79. 4% 80% 73% log rank p=0. 37 61. 9% log rank p=0. 13 Lammer et al. CVIR 2014
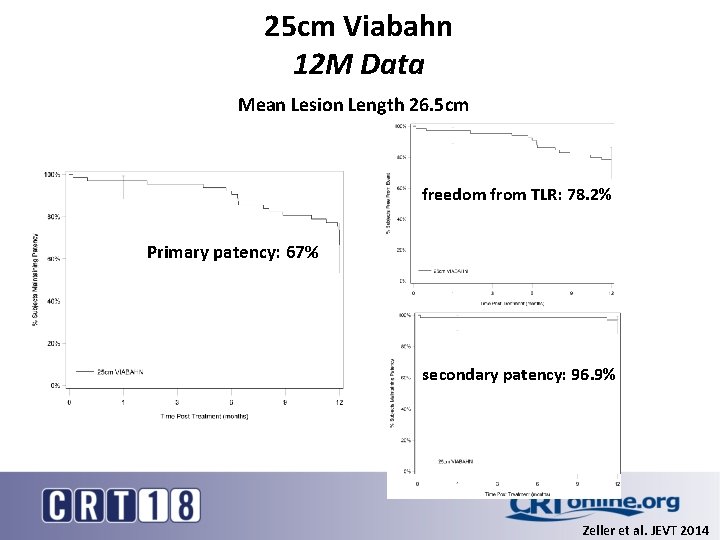
25 cm Viabahn 12 M Data Mean Lesion Length 26. 5 cm freedom from TLR: 78. 2% Primary patency: 67% secondary patency: 96. 9% Zeller et al. JEVT 2014
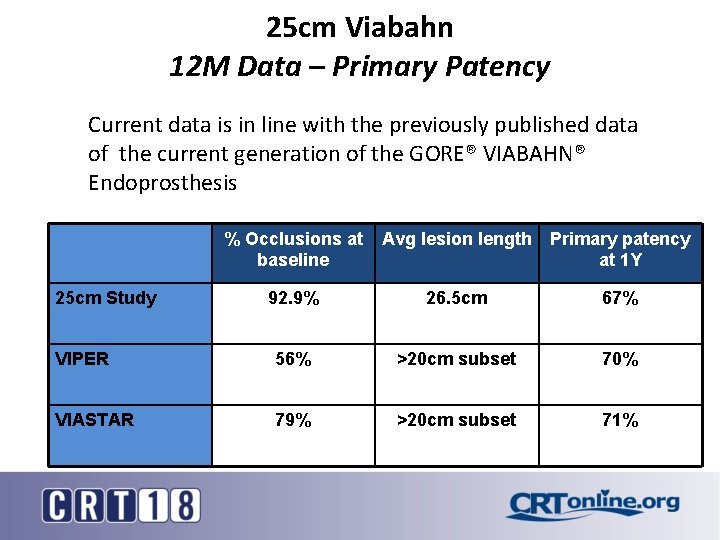
25 cm Viabahn 12 M Data – Primary Patency Current data is in line with the previously published data of the current generation of the GORE® VIABAHN® Endoprosthesis 25 cm Study % Occlusions at Avg lesion length baseline Primary patency at 1 Y 92. 9% 26. 5 cm 67% VIPER 56% >20 cm subset 70% VIASTAR 79% >20 cm subset 71%

Edge Stenosis - Neointimal Hyperplasia Achilles’ Heel of Viabahn Stent Graft (VIABAHN) Mechanical Barrier Potential Solution: Preparing the landing zone with DCB • Pore size provides a barrier to tissue ingrowth through the fabrique but not from the edges

Straight Stents Inhibit Shortening • Dominant force in vessel is axial compression • Up to 23% shortening in native vessel during flexion* • Straight stent may limit shortening to around 10%* • Risk of kinking at stent end or straight stent slack vessel kink Smouse BH, Nikanorov A, La. Flash D. Biomechanical forces in the femoropopliteal arterial segment. Endovasc Today. 2005; 4: 60 -66 15
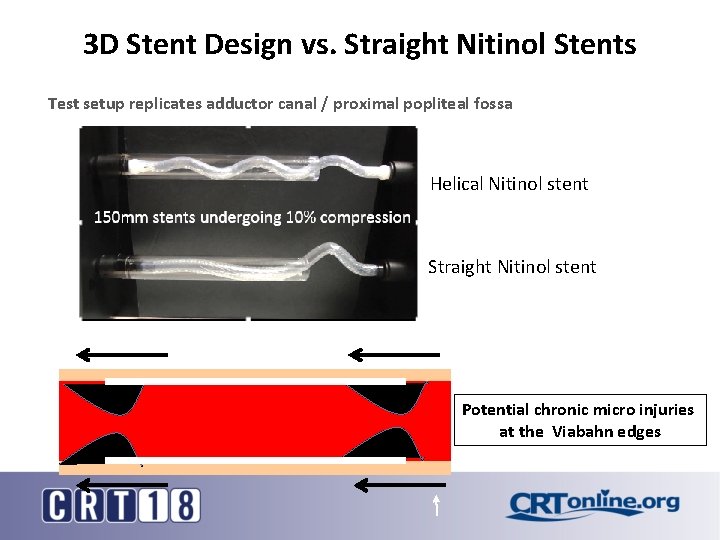
3 D Stent Design vs. Straight Nitinol Stents Test setup replicates adductor canal / proximal popliteal fossa Helical Nitinol stent 150 mm stents undergoing 10% compression Straight Nitinol stent Potential chronic micro injuries at the Viabahn edges
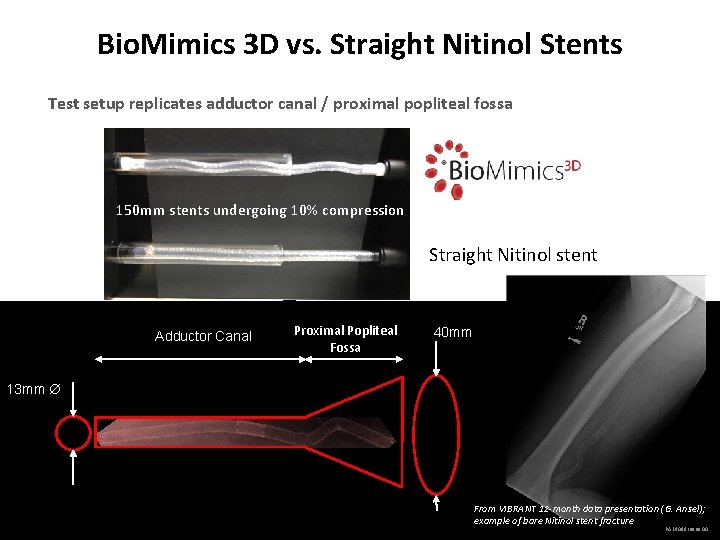
Bio. Mimics 3 D vs. Straight Nitinol Stents Test setup replicates adductor canal / proximal popliteal fossa 150 mm stents undergoing 10% compression Straight Nitinol stent Adductor Canal Proximal Popliteal Fossa 40 mm 13 mm From VIBRANT 12 -month data presentation (G. Ansel); example of bare Nitinol stent fracture PAM 098 Issue 00

DCB & Stentgrafts in Femoro-Popliteal Lesions Conclusions § DCB perform well in femoro-popliteal disease § Limitations of POBA § Dissection § Recoil § Thrombus § Viabahn endoprosthesis provides a mechanical barrier against tissue ingrowth § Limitation: Edge stenosis § Preparation of the landing zones of the Viabahn stentgraft might solve the limitations of both individual devices § Costs? § Pathophysiology?
- Slides: 18