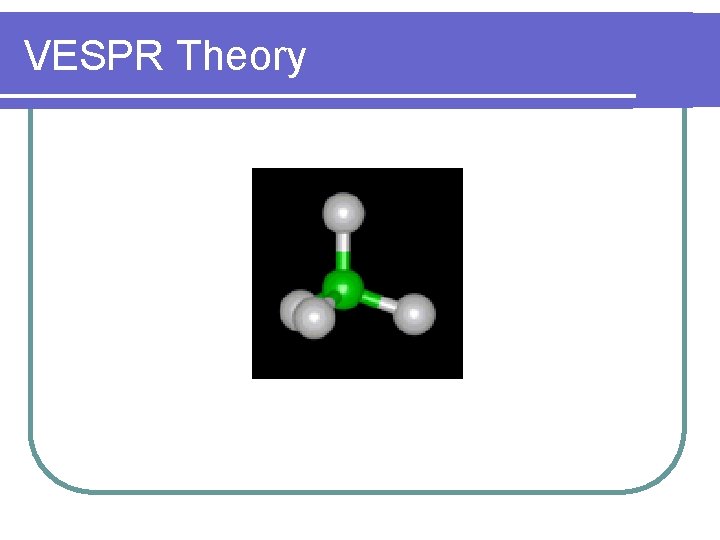
VESPR Theory VSEPR Theory Valence Shell Electron Pair



- Slides: 42

VESPR Theory

VSEPR Theory (Valence Shell Electron Pair Repulsion Theory) l A model for describing the shapes of molecules whose main postulate is that the structure around a given atom is determined by minimizing the electron pair repulsion l Therefore, the electrons and elements bonded to the central atom want to be as far apart as possible l

VSEPR Steps Draw the Lewis structure for the molecule 2. Count the total number of things that are around the central atom to determine the electron pair geometry 3. Imagine that the lone pairs of electrons are invisible and describe the molecular shape 1.

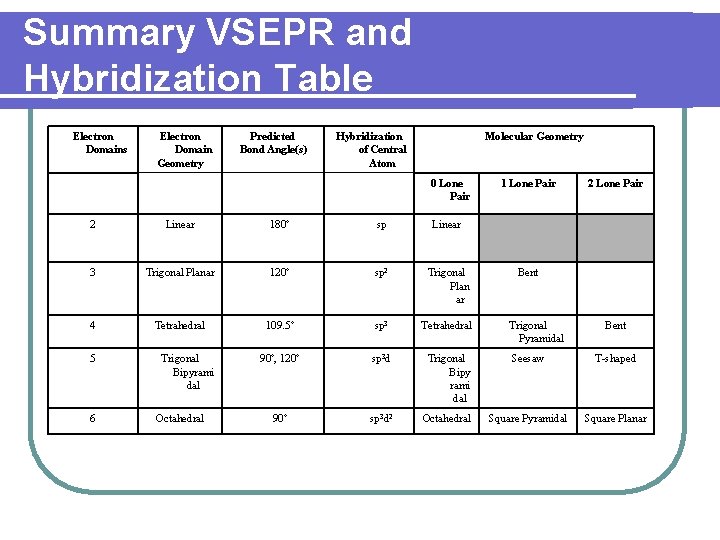
Summary VSEPR and Hybridization Table Electron Domains Electron Domain Geometry Predicted Bond Angle(s) Hybridization of Central Atom Molecular Geometry 0 Lone Pair 1 Lone Pair 2 Linear 180º sp Linear 3 Trigonal Planar 120º sp 2 Trigonal Plan ar 4 Tetrahedral 109. 5º sp 3 Tetrahedral 90º, 120º sp 3 d Trigonal Bipy rami dal Seesaw T-shaped 90º sp 3 d 2 Octahedral Square Pyramidal Square Planar 5 6 Trigonal Bipyrami dal Octahedral Bent Trigonal Pyramidal Bent

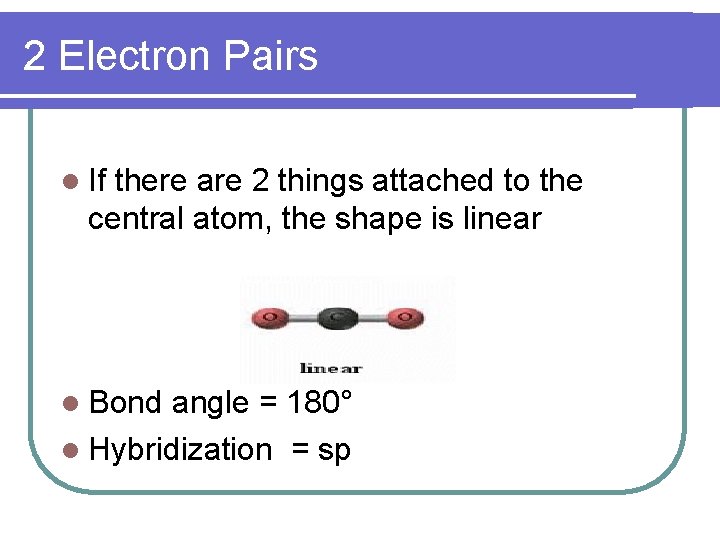
2 Electron Pairs l If there are 2 things attached to the central atom, the shape is linear l Bond angle = 180° l Hybridization = sp

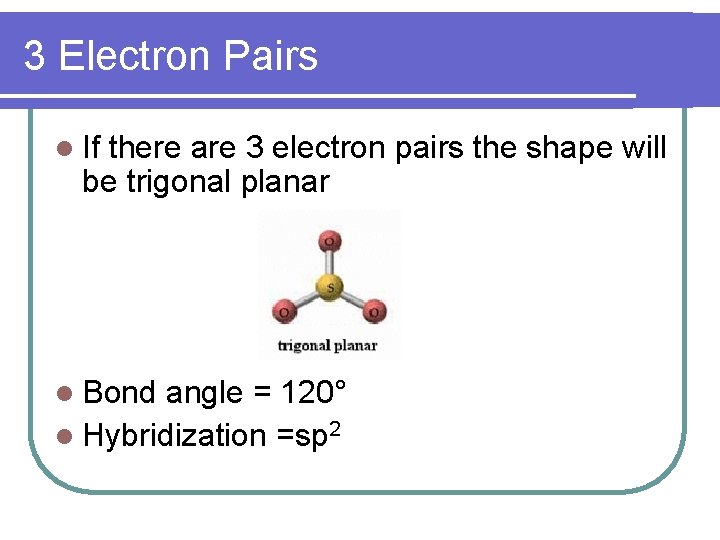
3 Electron Pairs l If there are 3 electron pairs the shape will be trigonal planar l Bond angle = 120° l Hybridization =sp 2

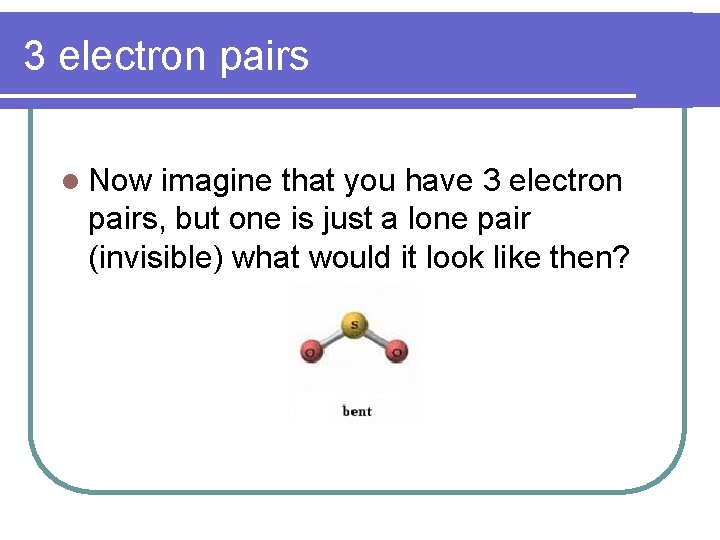
3 electron pairs l Now imagine that you have 3 electron pairs, but one is just a lone pair (invisible) what would it look like then?

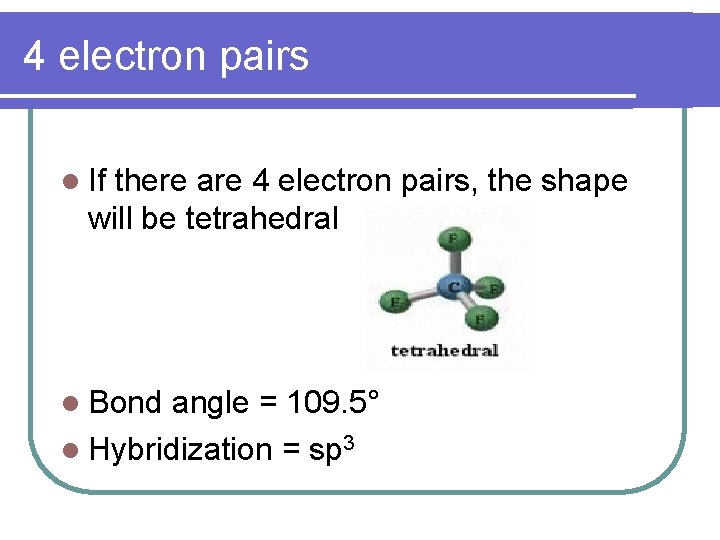
4 electron pairs l If there are 4 electron pairs, the shape will be tetrahedral l Bond angle = 109. 5° l Hybridization = sp 3

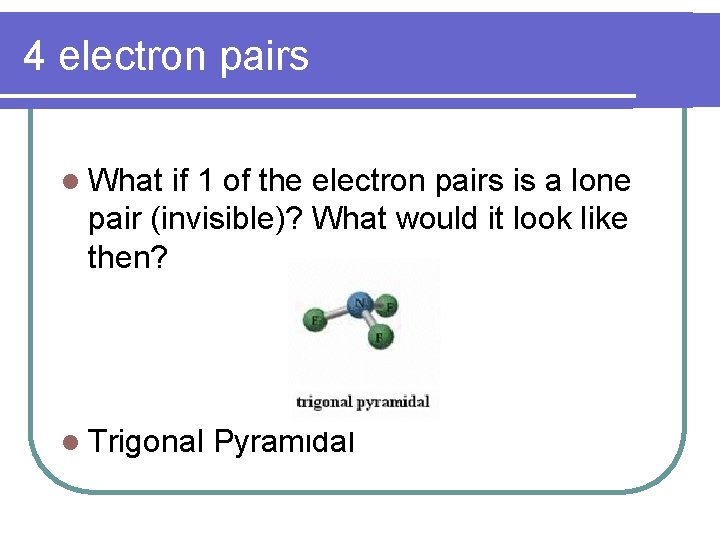
4 electron pairs l What if 1 of the electron pairs is a lone pair (invisible)? What would it look like then? l Trigonal Pyramidal

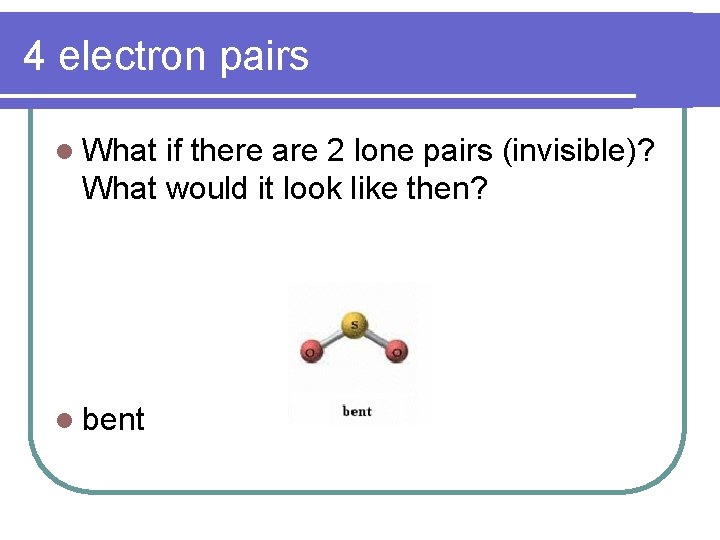
4 electron pairs l What if there are 2 lone pairs (invisible)? What would it look like then? l bent

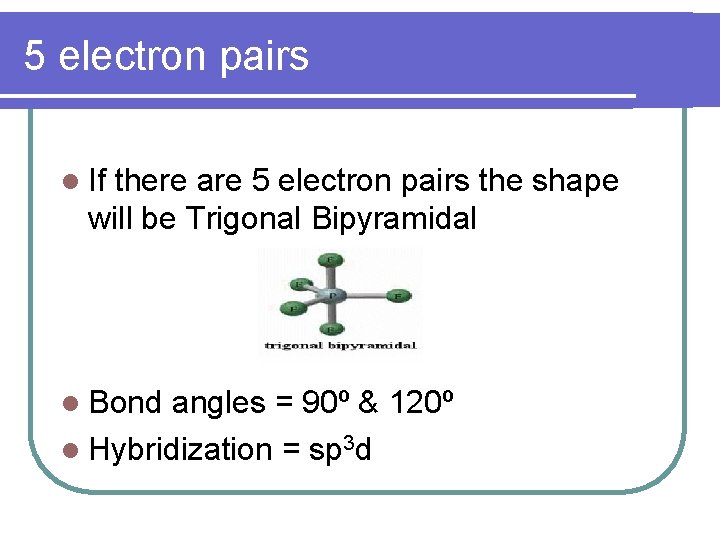
5 electron pairs l If there are 5 electron pairs the shape will be Trigonal Bipyramidal l Bond angles = 90º & 120º l Hybridization = sp 3 d

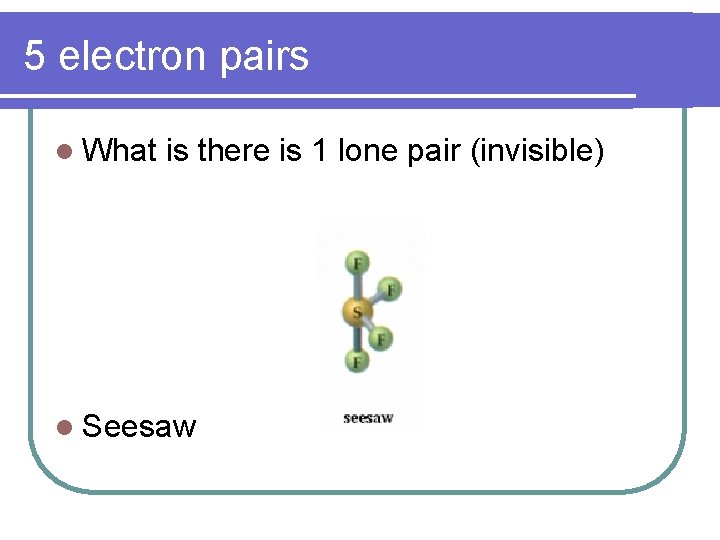
5 electron pairs l What is there is 1 lone pair (invisible) l Seesaw

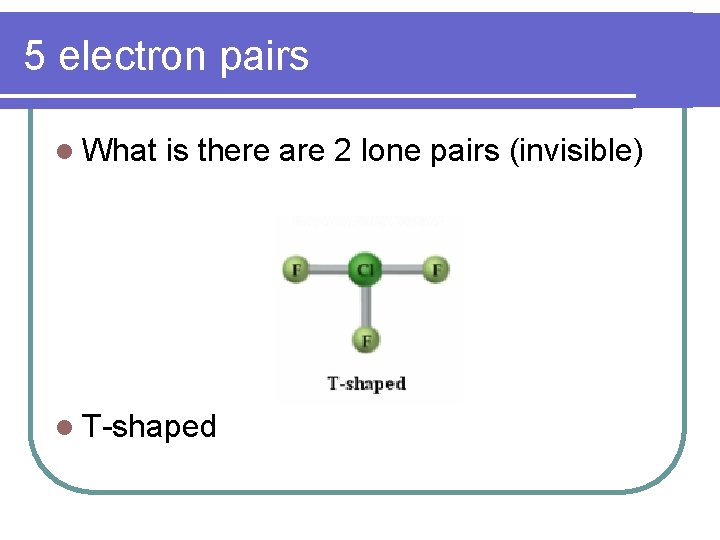
5 electron pairs l What is there are 2 lone pairs (invisible) l T-shaped

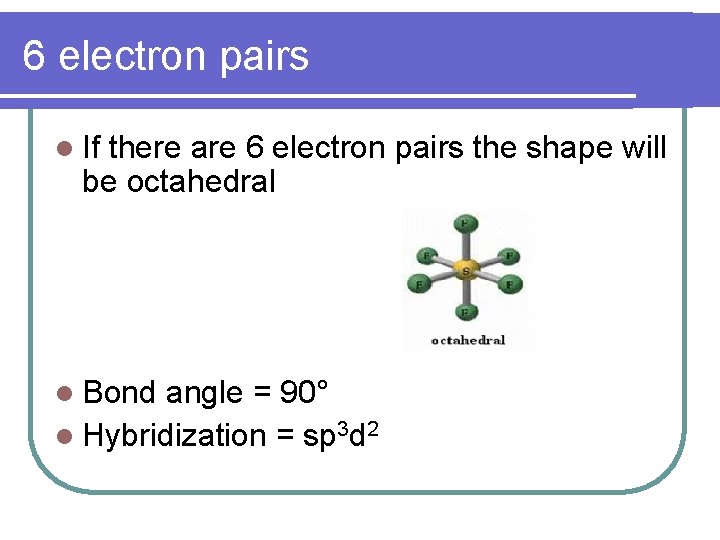
6 electron pairs l If there are 6 electron pairs the shape will be octahedral l Bond angle = 90° l Hybridization = sp 3 d 2

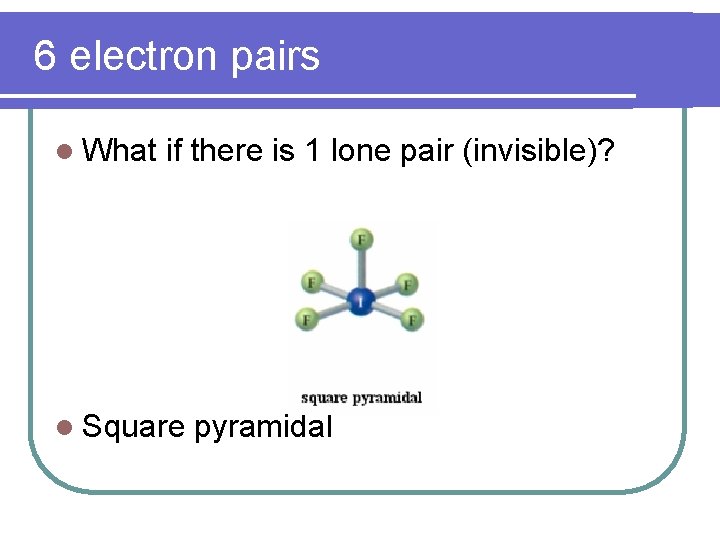
6 electron pairs l What if there is 1 lone pair (invisible)? l Square pyramidal

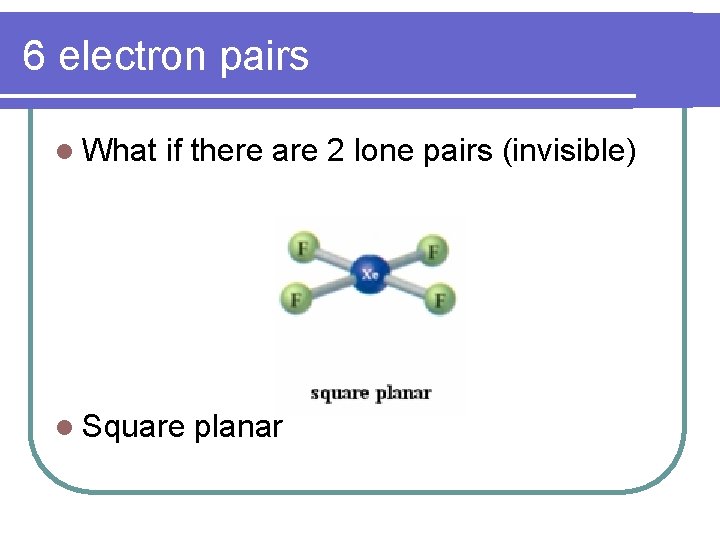
6 electron pairs l What if there are 2 lone pairs (invisible) l Square planar

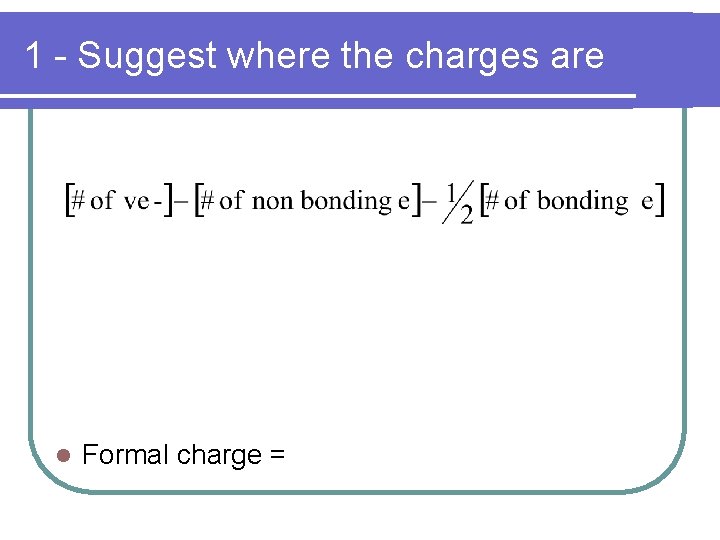
Formal Charge Formal charges can be used in 1 of 2 ways… 1. Suggest where the charges are 2. Help select the most plausible structure from a set of resonance structures l

1 - Suggest where the charges are l Formal charge =

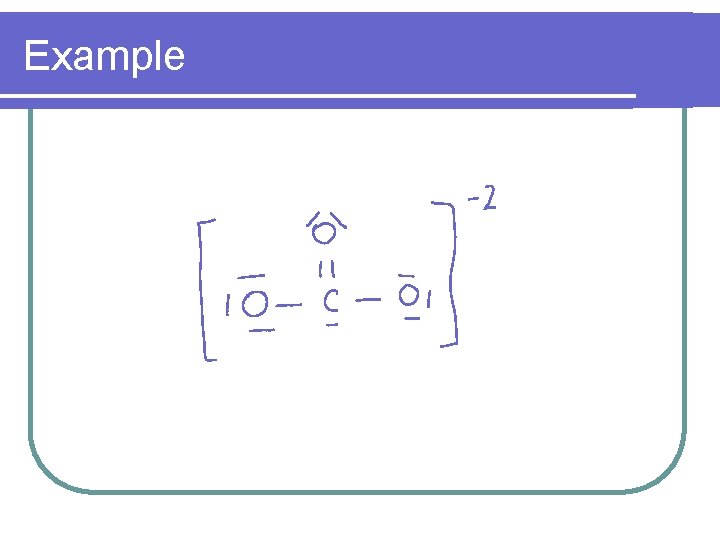
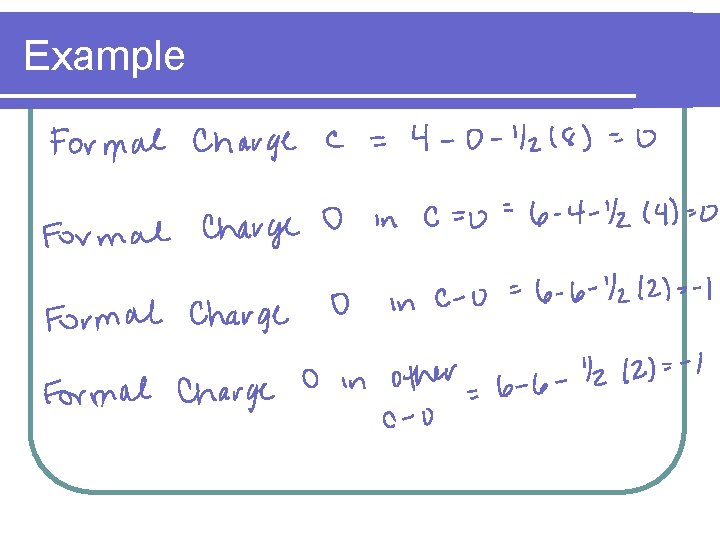
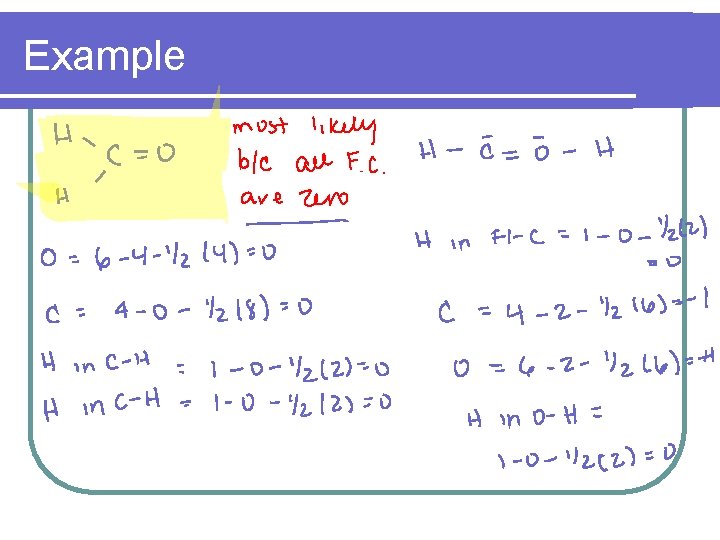
Example l Calculate the formal charge on each element in the carbonate ion l CO 3 2 -

Example

Example

Example l The sum of the formal charges of the individual charges equals the formal charge on the molecule or ion l The formal charge for carbonate = l 0 + -1 = -2

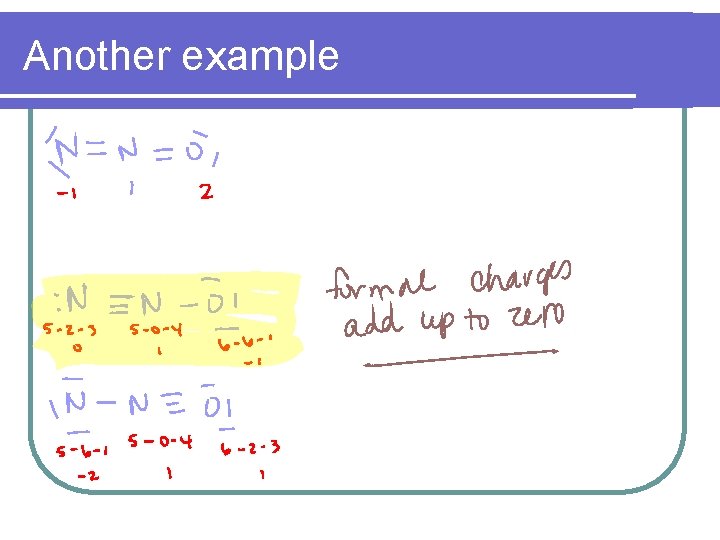
2 - Help select the most plausible structure from a set of resonance structures l l l When choosing the most likely resonance structure Most likely – All formal charges are zero Next likely – All formal charges add up to zero Next likely – Formal charges add up to the lowest possible charge Next likely – Negative charge is on most electronegative atom

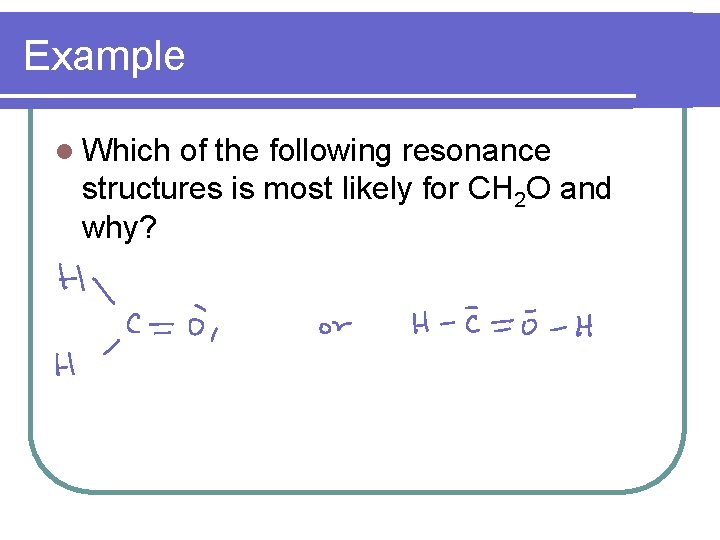
Example l Which of the following resonance structures is most likely for CH 2 O and why?

Example

Another Example l Which N 2 O? is the most likely structure for

Another example

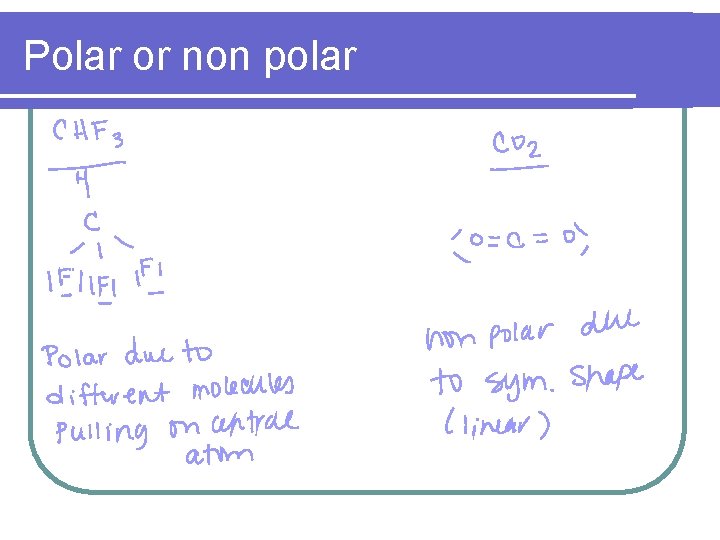
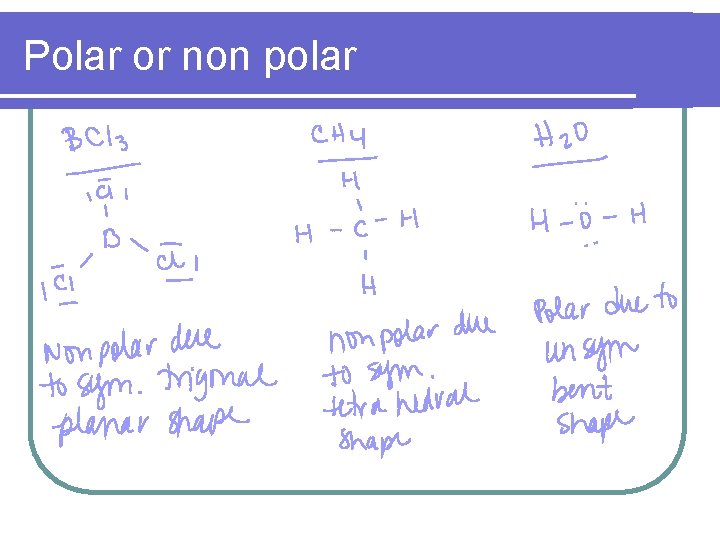
Polar bonds & polar molecules (Dipole or non dipole) l In order for a substance to be polar, the bonds within the molecule must carry different charges and cannot cancel out due to symmetry

Polar or non polar l CHF 3 l CO 2 l BCl 3 l CH 4 l H 2 O

Polar or non polar

Polar or non polar

Rule for solubility l Like dissolves like l Polar will dissolve in polar l Non polar will dissolve in non polar

Bonding l Intramolecular forces – bonding within molecule (ionic or covalent) l Intermolecular forces – bonding between molecules

Intermolecular Bonding 2 factors determine if a substance is a solid, liquid, or a gas: 1. Kinetic energy 2. Intermolecular forces holding the particles together l

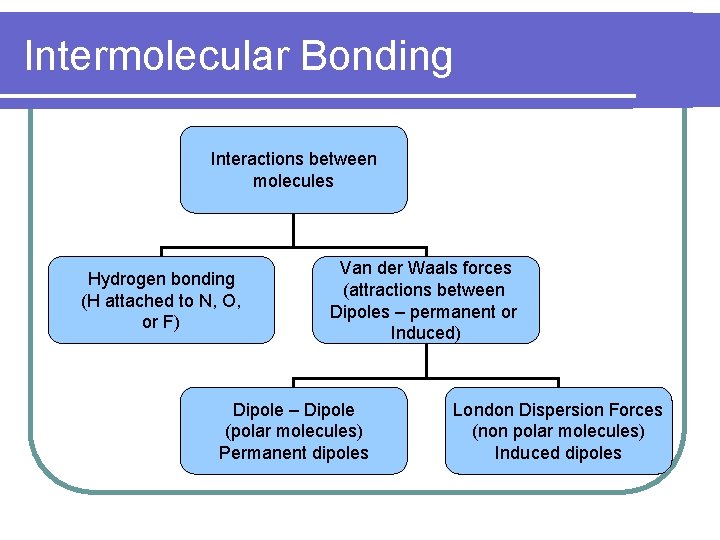
Intermolecular Bonding Interactions between molecules Hydrogen bonding (H attached to N, O, or F) Van der Waals forces (attractions between Dipoles – permanent or Induced) Dipole – Dipole (polar molecules) Permanent dipoles London Dispersion Forces (non polar molecules) Induced dipoles

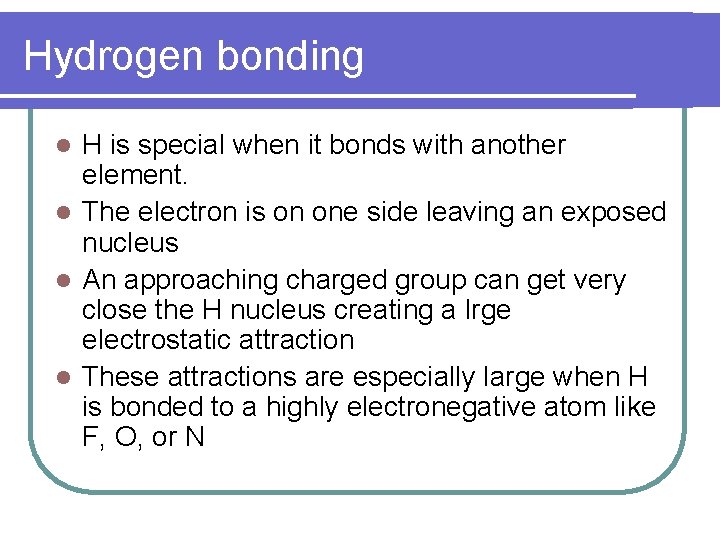
Hydrogen bonding H is special when it bonds with another element. l The electron is on one side leaving an exposed nucleus l An approaching charged group can get very close the H nucleus creating a lrge electrostatic attraction l These attractions are especially large when H is bonded to a highly electronegative atom like F, O, or N l

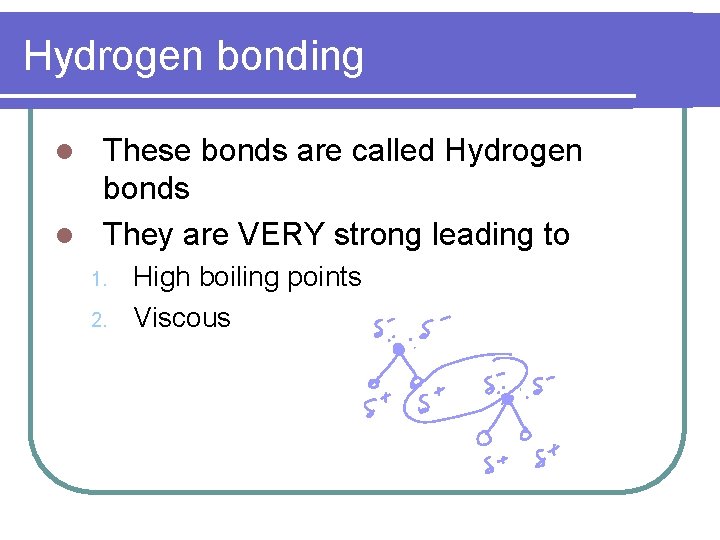
Hydrogen bonding These bonds are called Hydrogen bonds l They are VERY strong leading to l 1. 2. High boiling points Viscous

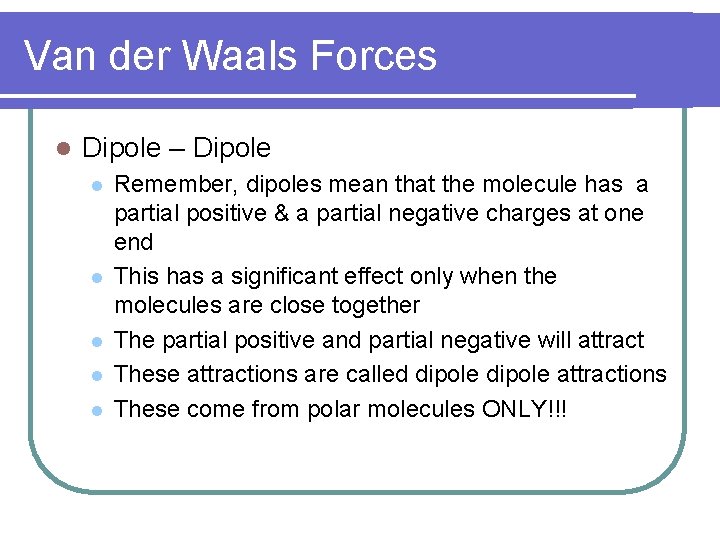
Van der Waals Forces l Dipole – Dipole l l l Remember, dipoles mean that the molecule has a partial positive & a partial negative charges at one end This has a significant effect only when the molecules are close together The partial positive and partial negative will attract These attractions are called dipole attractions These come from polar molecules ONLY!!!

London Dispersion forces l Small electrostatic forces caused by the movement of the electron in molecules that n=have no permanent dipole l In all molecules – polar & non polar l Heavier atoms stronger LDF because valence electrons are further apart in larger molecules & held less tightly so they can more easily form temporary dipoles

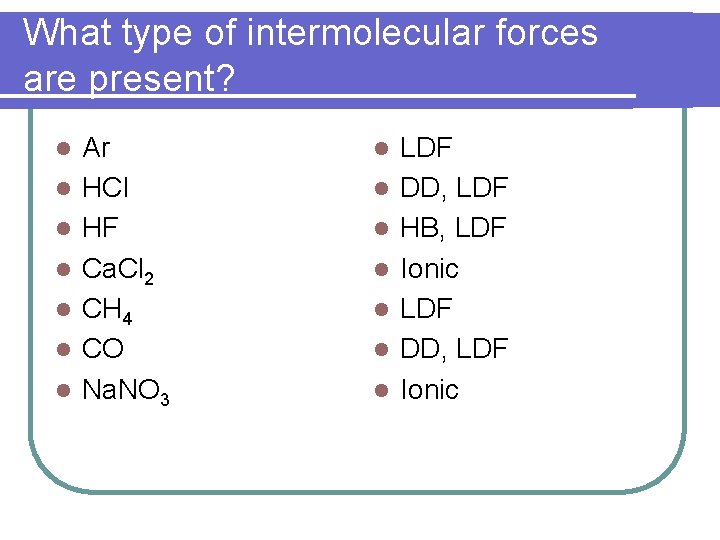
What type of intermolecular forces are present? l l l l Ar HCl HF Ca. Cl 2 CH 4 CO Na. NO 3 l l l l LDF DD, LDF HB, LDF Ionic LDF DD, LDF Ionic

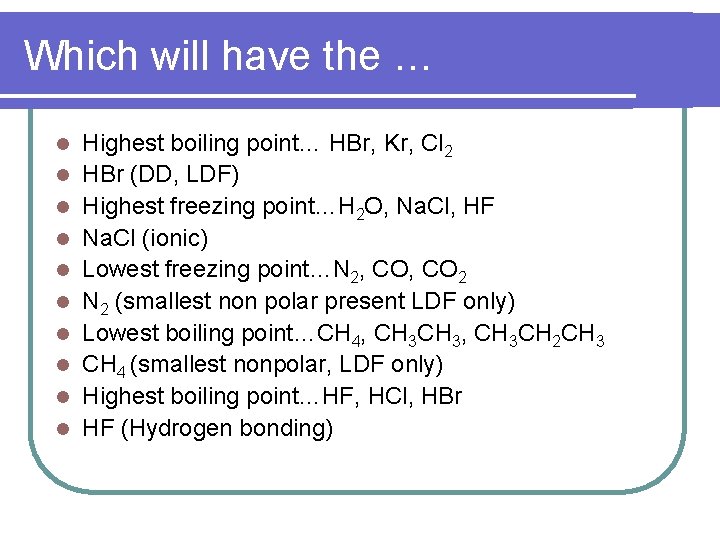
Which will have the … l l l l l Highest boiling point… HBr, Kr, Cl 2 HBr (DD, LDF) Highest freezing point…H 2 O, Na. Cl, HF Na. Cl (ionic) Lowest freezing point…N 2, CO 2 N 2 (smallest non polar present LDF only) Lowest boiling point…CH 4, CH 3, CH 3 CH 2 CH 3 CH 4 (smallest nonpolar, LDF only) Highest boiling point…HF, HCl, HBr HF (Hydrogen bonding)

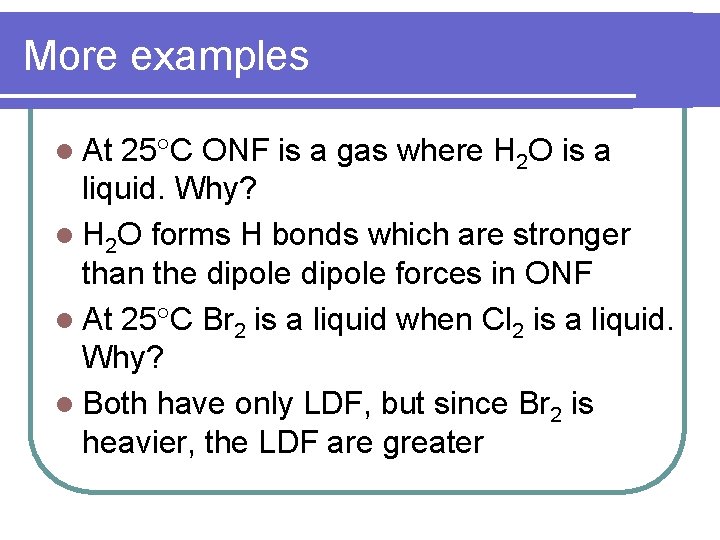
More examples l At 25 C ONF is a gas where H 2 O is a liquid. Why? l H 2 O forms H bonds which are stronger than the dipole forces in ONF l At 25 C Br 2 is a liquid when Cl 2 is a liquid. Why? l Both have only LDF, but since Br 2 is heavier, the LDF are greater