Version 4 1 2013 Chemistry NCEA L 2

![Complex Ions tetrahydroxy plumbate(II) [Pb(OH)4]2 tetrahydroxyzincate(II) [Zn(OH)4]2 Step one – add Na. OH Pb Complex Ions tetrahydroxy plumbate(II) [Pb(OH)4]2 tetrahydroxyzincate(II) [Zn(OH)4]2 Step one – add Na. OH Pb](https://slidetodoc.com/presentation_image_h2/42081e38f11d429f402b8479c236b636/image-27.jpg)
![Complex Ions tetrahydroxyaluminate(III) [Al(OH)4] Step one – add Na. OH Al 3+ + 3 Complex Ions tetrahydroxyaluminate(III) [Al(OH)4] Step one – add Na. OH Al 3+ + 3](https://slidetodoc.com/presentation_image_h2/42081e38f11d429f402b8479c236b636/image-28.jpg)
![Complex Ions tetraamminezinc(II) [Zn(NH 3)4]2+ tetraamminecopper(II) [Cu(NH 3)4]2+ NH 3 + H 2 O Complex Ions tetraamminezinc(II) [Zn(NH 3)4]2+ tetraamminecopper(II) [Cu(NH 3)4]2+ NH 3 + H 2 O](https://slidetodoc.com/presentation_image_h2/42081e38f11d429f402b8479c236b636/image-29.jpg)
![Complex Ions diamminesilver(I) [Ag(NH 3)2]+ NH 3 + H 2 O NH 4+ + Complex Ions diamminesilver(I) [Ag(NH 3)2]+ NH 3 + H 2 O NH 4+ +](https://slidetodoc.com/presentation_image_h2/42081e38f11d429f402b8479c236b636/image-30.jpg)
- Slides: 41

Version 4. 1 2013 Chemistry NCEA L 2 2. 2 Identify Ions 2013 Chemistry NCEA L 2 2. 1 Identify Ions GZ SJ Gaze

Achievement Standard 91162 Carry out procedures to identify ions involves collecting primary data and using these observations to identify ions in a solution using a procedure provided. Carry out procedures to justify the identification of ions also includes writing balanced equations for all the reactions where precipitates are formed. Carry out procedures to comprehensively justify the identification of ions also includes interpreting observations by recognising the formation of complex ions and writing balanced equations for these reactions. Identification of ions must be supported by experimental observations and identification of all precipitates formed. Ions to be identified will be limited to: Ag+, Al 3+, Ba 2+, Cu 2+, Fe 3+, Mg 2+, Pb 2+, Na+, Zn 2+, Cl–, CO 32–, I– , NO 3–, OH–, SO 42–. (Na+ and NO 3– are identified by a process of elimination. ) Complex ions may include [Fe. SCN]2+ and those formed when OH–(aq) or NH 3(aq) react with cations listed above, such as [Ag(NH 3)2]+, [Al(OH)4]–, [Pb(OH)4]2–, [Zn(NH 3)4]2+, [Cu(NH 3)4]2+. 2013 Chemistry NCEA L 2 2. 1 Identify Ions GZ

Key Concepts >Ions – Anions ( ve) and Cations (+ve) >Ionic solution equations – breaking compounds into their ions >Formal equations – adding reactants to get products >Forming precipitates (insoluble ionic compounds) >Solubility rules – which compounds are soluble or insoluble >Ionic equations leaving out spectator ions, solubility grids. >Testing for ions based on whether they precipitate or not and what colour the precipitate is. >Flow charts to help testing >Reagents – chemicals to help bring about a reaction >Complex ions – ionic compounds with a charge >Practice testing for ions 2013 Chemistry NCEA L 2 2. 1 Identify Ions GZ

Ions are charged particles. Ions form when atoms gain or lose electrons. Ions are formed when an atom loses or gains electrons. Elements are most stable when the outer shell (valence shell) is full. Elements can lose or gain electrons when they react with other chemicals to form ions. 2013 Chemistry NCEA L 2 2. 1 Identify Ions GZ

Anions and Cations Atoms that lose electrons form positively charged ions, or cations. Atoms that gain electrons form negatively charged ions, or anions. Cation (Cat) Anion (an Iron) + Metals lose electrons to form Cations. They have 1 3 electrons in their outside shell Non-Metals gain electrons to form Anions. They have 7 8 electrons in their outside shell.

Anions and Cations Ions are atoms or groups of atoms with electrical charge. Elements are most stable when the outer shell (valence shell) is full. Elements can lose or gain electrons when they react with other chemicals to form ions. Cation Sodium (Na) 11+ Sodium now becomes the sodium ion Na+ Anion Chlorine (Cl) 17+ Chlorine now becomes the chlorine ion Cl

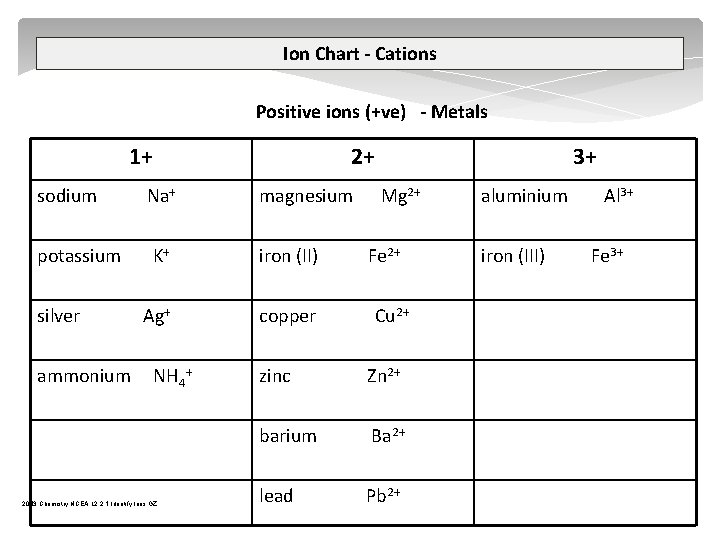
Ion Chart - Cations Positive ions (+ve) - Metals 1+ 2+ sodium Na+ magnesium potassium K+ iron (II) Ag+ copper silver ammonium NH 4+ 2013 Chemistry NCEA L 2 2. 1 Identify Ions GZ 3+ Mg 2+ Fe 2+ Cu 2+ zinc Zn 2+ barium Ba 2+ lead Pb 2+ aluminium iron (III) Al 3+ Fe 3+

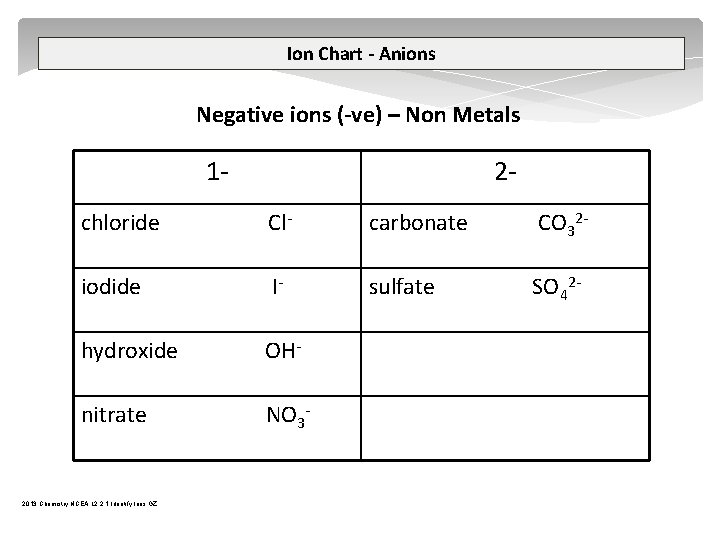
Ion Chart - Anions Negative ions (-ve) – Non Metals 1 2 chloride Cl carbonate CO 32 iodide I sulfate SO 42 hydroxide OH nitrate NO 3 2013 Chemistry NCEA L 2 2. 1 Identify Ions GZ

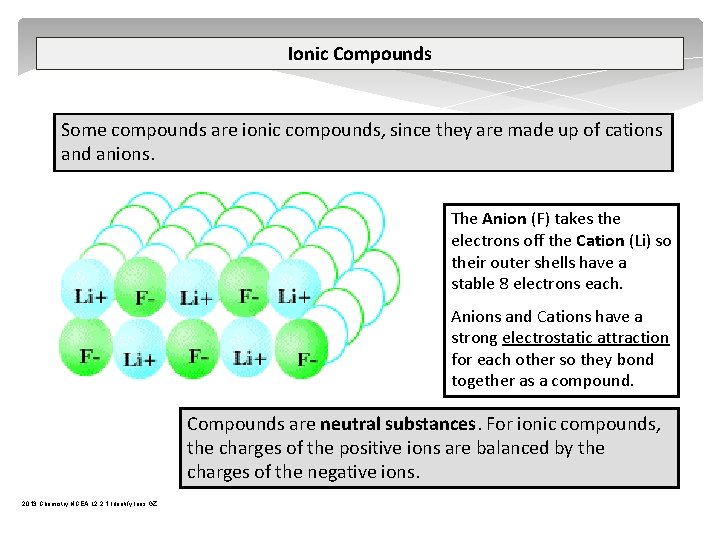
Ionic Compounds Some compounds are ionic compounds, since they are made up of cations and anions. The Anion (F) takes the electrons off the Cation (Li) so their outer shells have a stable 8 electrons each. Anions and Cations have a strong electrostatic attraction for each other so they bond together as a compound. Compounds are neutral substances. For ionic compounds, the charges of the positive ions are balanced by the charges of the negative ions. 2013 Chemistry NCEA L 2 2. 1 Identify Ions GZ

Ions in solution The ions of the ionic compound are in an aqueous solution – dissolved in water. They are therefore free moving and available to form bonds with other ions Soluble dissolves in water form a solution e. g. Na. Cl(s) → Na+(aq) + Cl (aq) Solubility = 35 g/100 g Sparingly Soluble slightly soluble e. g. Calcium hydroxide Solubility = 0. 1 g/100 g Insoluble extremely solubility e. g. Silver chloride 2013 Chemistry NCEA L 2 2. 1 Identify Ions GZ Solubility = 0. 0002 g/100 g

Naming Chemical Formula Compounds are represented by chemical formulas. If the compound is formed between a Metal cation (+ve) and a Non Metal anion ( ve), then the compound name joins the two names together with the metal name first. Sodium + hydroxide You will be given an ion chart with the formula of the ions but you will need to remember the names. 2013 Chemistry NCEA L 2 2. 1 Identify Ions GZ

Ionic Solution equations Ionic compounds in solution break down into their ions. Na. Cl(s) dissolving Na+(aq) + Cl-(aq) Write ionic solution equations for the following a. potassium hydroxide b. sodium nitrate c. magnesium chloride d. copper sulfate e. sodium carbonate f. aluminium nitrate 2013 Chemistry NCEA L 2 2. 1 Identify Ions GZ

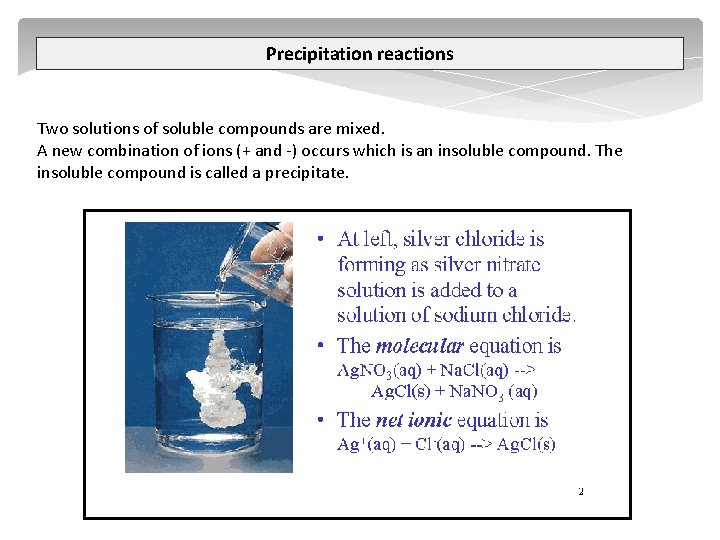
Precipitation reactions Two solutions of soluble compounds are mixed. A new combination of ions (+ and ) occurs which is an insoluble compound. The insoluble compound is called a precipitate.

Solubility Rules Some ions will form precipitates and are insoluble. Other ions will not form precipitates and are soluble. Ion Rule Exceptions nitrate soluble chloride, iodide soluble silver and lead sulfate soluble lead, calcium, barium carbonate insoluble sodium, potassium ammonium hydroxides insoluble sodium, potassium sodium potassium ammonium all soluble

Solubility Rules Some ions will form precipitates and are insoluble. Other ions will not form precipitates and are soluble. Ion Rule NO 3 soluble Cl , I soluble Ag+ and Pb 2+ SO 42 CO 32 soluble insoluble Pb 2+ , Ca 2+ , Ba 2+ Na+, K+ and NH 4+ OH Na+, K+, NH 4+ insoluble all soluble Na+, K+ 2013 Chemistry NCEA L 2 2. 1 Identify Ions GZ Exceptions

Solubility Grids When adding one ionic solution to another we use a solubility grid to decide if a precipitate has formed or not. e. g. Ca. Cl 2(aq) + Na 2 CO 3(aq) g Ca 2+ 2 Cl 1 - Na+ ? ? CO 32? - The Na+ and the Cl ions are dissolved in solution in the beginning and remain in solution at the end. They are not involved in the precipitation reaction so they are known as spectator ions. They do not need to be writen in the equations for the reactions. 2013 Chemistry NCEA L 2 2. 1 Identify Ions GZ

Solubility For each ionic compound decide whether it is soluble or not. If insoluble, write the formula of the solid down. If soluble, write the formulae of the ions present in a solution of the compound. a. sodium chloride c. ammonium nitrate e. potassium carbonate g. sodium sulfate i. silver iodide k. zinc nitrate m. barium sulfate o. zinc hydroxide q. sodium bicarbonate s. iron (II) sulfate u. calcium carbonate 2013 Chemistry NCEA L 2 2. 1 Identify Ions GZ b. copper hydroxide d. barium sulfate f. silver chloride h. magnesium nitrate j. aluminum hydroxide l. copper (II) carbonate n. iron (III) chloride p. potassium chloride r. sodium bromide t. ammonium carbonate v. aluminum oxide

Balancing Equations Balanced equations must have the same number of atoms on each side of the equation i. e. reactants and products. 2 Na Na + Na 2013 Chemistry NCEA L 2 2. 1 Identify Ions GZ 2 H 2 O H H O = H 2 Na. OH O Na Na + H 2 H HH

Equations Ca. Cl 2(aq) + Na 2 CO 3(aq) Ca. CO 3(s) + 2 Na. Cl(aq) Use solubility grids and solubility rules to complete the following equations Write formal equations and ionic equations where a ppt reaction occurs. a. b. c. d. e. f. g. h. i. Ba(NO 3)2 (aq) + Na 2 SO 4(aq) Cu(NO 3)2(aq) + Na. OH (aq) Ag. NO 3 (aq) + K 2 CO 3(aq) Cu(NO 3)2(aq) + Mg. Cl 2(aq) Ca(NO 3)2(aq) + (NH 4)2 SO 4(aq) KCl(aq) + Ag. NO 3(aq) NH 4 OH(aq) + Fe. Cl 3(aq) Cu. SO 4(aq) + Na. OH(aq) Ag. NO 3(aq) + KI(aq) 2013 Chemistry NCEA L 2 2. 1 Identify Ions GZ

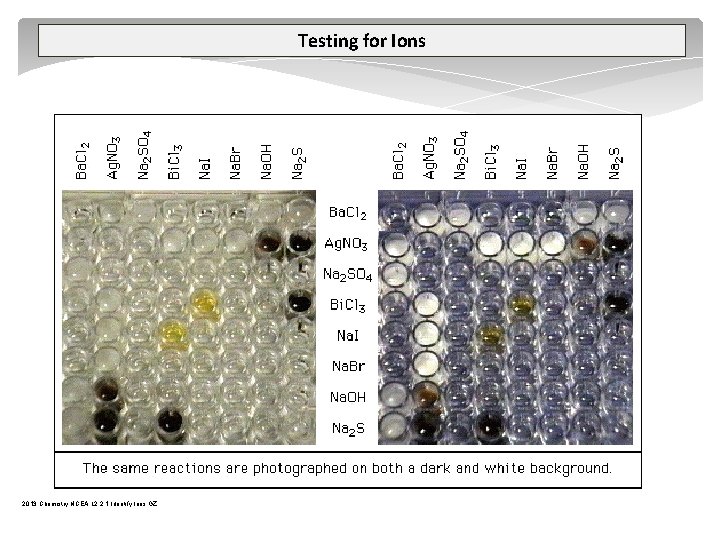
Testing for Ions 2013 Chemistry NCEA L 2 2. 1 Identify Ions GZ

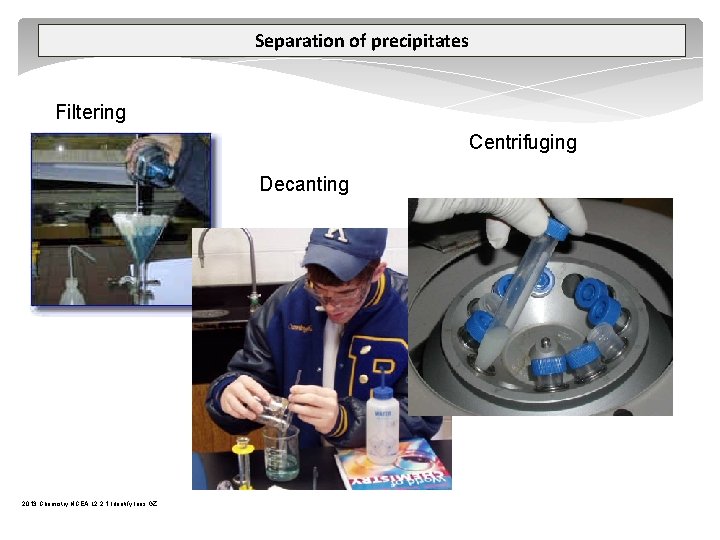
Separation of precipitates Filtering Centrifuging Decanting 2013 Chemistry NCEA L 2 2. 1 Identify Ions GZ

Important reagents Ammonia / Ammonium Hydroxide solution Ammonia reacts with water in a reversible reaction NH 3 (aq) + H 2 O(l) NH 4+(aq) + OH-(aq) The same solution contains two reactants OH and NH 3 The OH (aq) concentration is quite low, but is important in the reactions of ammonia solutions 2013 Chemistry NCEA L 2 2. 1 Identify Ions GZ

Ion Equations Under each step of the flow chart that produces a precipitate the correct ionic equation will need to be written. Use your ion chart your solubillty rules and solubility grids For example Add 2 drops Na. OH (aq). 1. No ppt. NH 4+, Na+ Add 1 m. L Na. OH (aq) Heat. Test for gas with wet red litmus. No reaction. Na+ turns litmus blue/smell of ammonia, (fishy) NH 4+ + OH-(g) NH 3(g) + H 2 O 2013 Chemistry NCEA L 2 2. 1 Identify Ions GZ

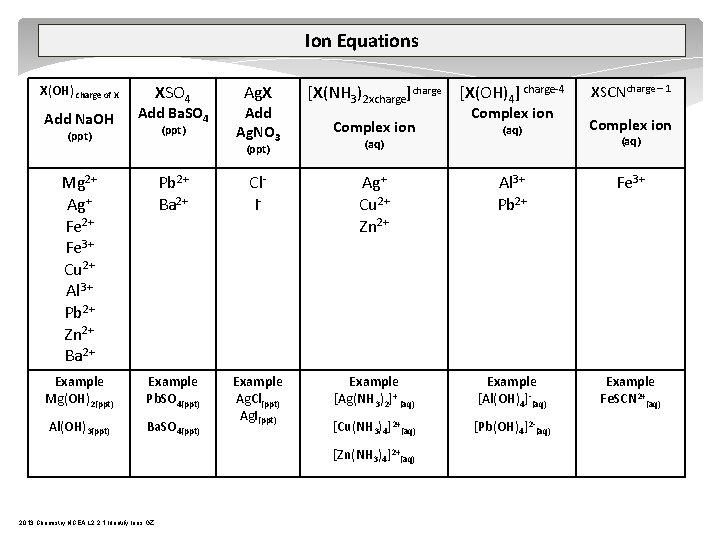
Ion Equations XSO 4 X(OH) charge of X Add Na. OH Add Ba. SO 4 (ppt) Ag. X Add Ag. NO 3 [X(NH 3)2 xcharge]charge [X(OH)4] charge 4 XSCNcharge – 1 Complex ion (aq) Complex ion (ppt) (aq) Complex ion (aq) Mg 2+ Ag+ Fe 2+ Fe 3+ Cu 2+ Al 3+ Pb 2+ Zn 2+ Ba 2+ Pb 2+ Ba 2+ Cl I Ag+ Cu 2+ Zn 2+ Al 3+ Pb 2+ Fe 3+ Example Mg(OH)2(ppt) Example Pb. SO 4(ppt) Example [Ag(NH 3)2]+ (aq) Example [Al(OH)4] (aq) Example Fe. SCN 2+(aq) Al(OH)3(ppt) Ba. SO 4(ppt) Example Ag. Cl(ppt) Ag. I(ppt) [Cu(NH 3)4]2+(aq) [Pb(OH)4]2 (aq) [Zn(NH 3)4]2+(aq) 2013 Chemistry NCEA L 2 2. 1 Identify Ions GZ

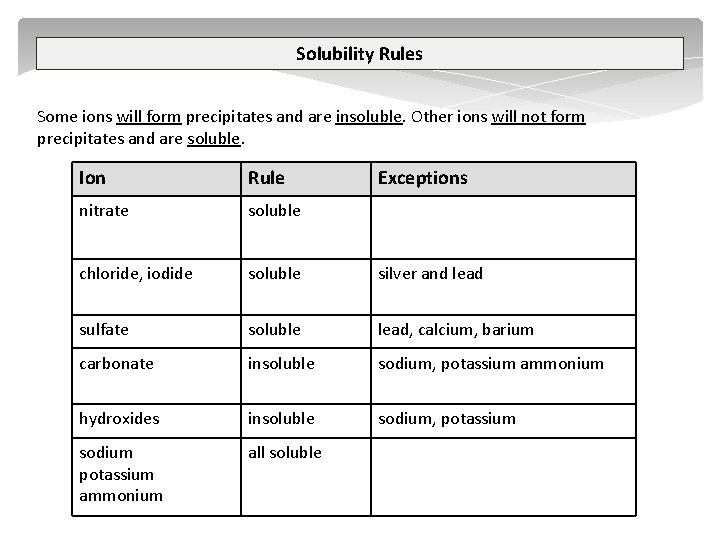
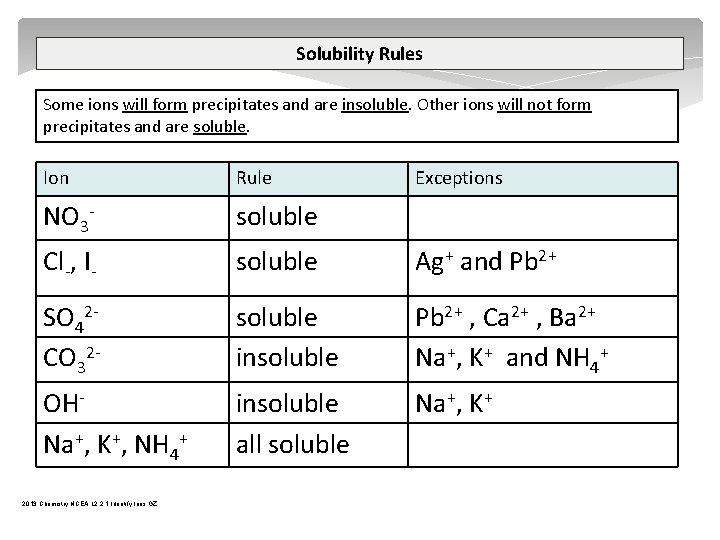
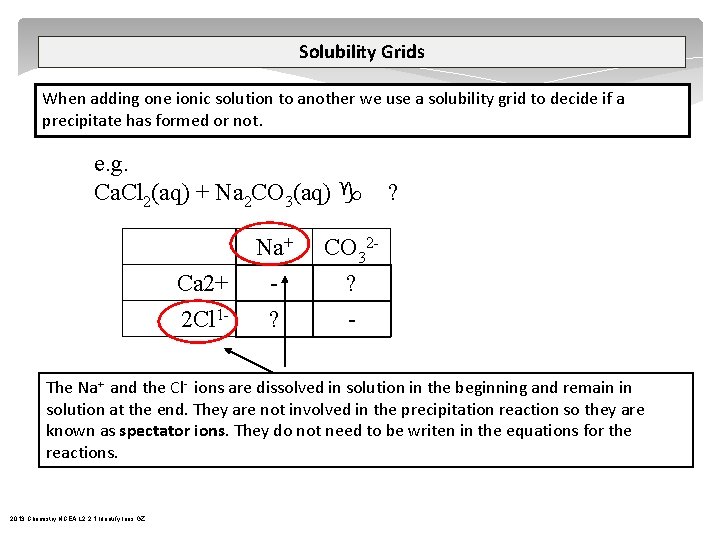
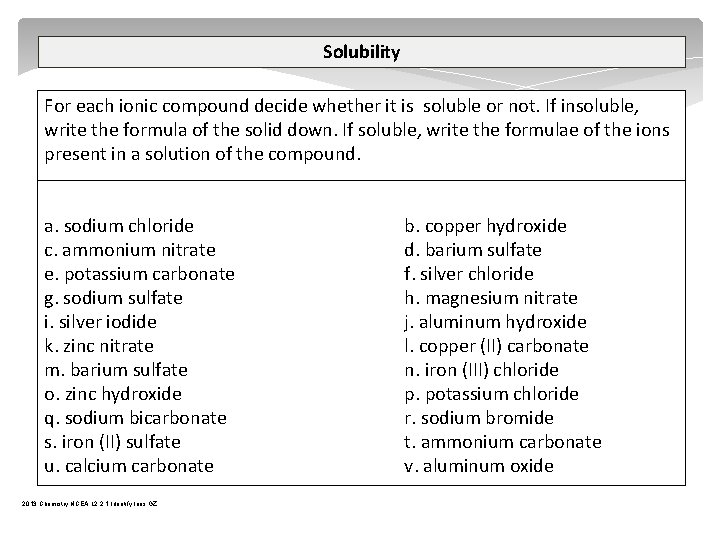
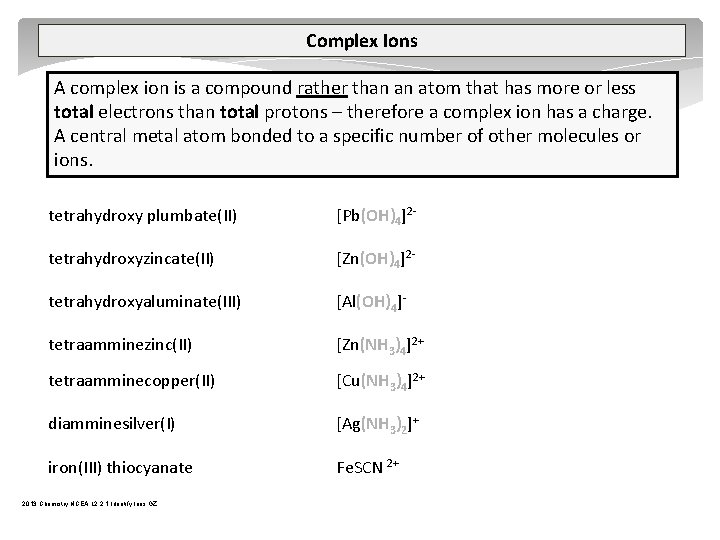
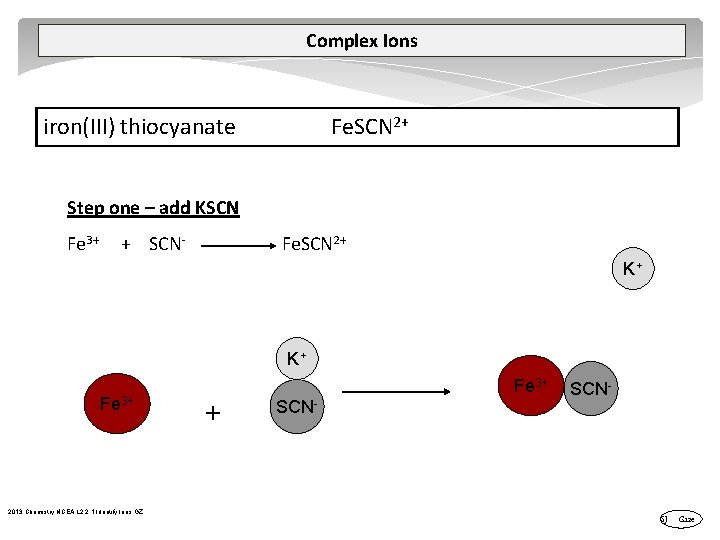
Complex Ions A complex ion is a compound rather than an atom that has more or less total electrons than total protons – therefore a complex ion has a charge. A central metal atom bonded to a specific number of other molecules or ions. tetrahydroxy plumbate(II) [Pb(OH)4]2 tetrahydroxyzincate(II) [Zn(OH)4]2 tetrahydroxyaluminate(III) [Al(OH)4] tetraamminezinc(II) [Zn(NH 3)4]2+ tetraamminecopper(II) [Cu(NH 3)4]2+ diamminesilver(I) [Ag(NH 3)2]+ iron(III) thiocyanate Fe. SCN 2+ 2013 Chemistry NCEA L 2 2. 1 Identify Ions GZ

2013 Chemistry NCEA L 2 2. 1 Identify Ions GZ
![Complex Ions tetrahydroxy plumbateII PbOH42 tetrahydroxyzincateII ZnOH42 Step one add Na OH Pb Complex Ions tetrahydroxy plumbate(II) [Pb(OH)4]2 tetrahydroxyzincate(II) [Zn(OH)4]2 Step one – add Na. OH Pb](https://slidetodoc.com/presentation_image_h2/42081e38f11d429f402b8479c236b636/image-27.jpg)
Complex Ions tetrahydroxy plumbate(II) [Pb(OH)4]2 tetrahydroxyzincate(II) [Zn(OH)4]2 Step one – add Na. OH Pb 2+ + 2 OH Pb(OH)2 Step two – add excess Na. OH Pb(OH)2 + 2 OH [Pb(OH)4]2 OH- OHOH- Pb 2+ 2013 Chemistry NCEA L 2 2. 1 Identify Ions GZ OH- + OH- Pb 2+ OH- OHOH- SJ Gaze
![Complex Ions tetrahydroxyaluminateIII AlOH4 Step one add Na OH Al 3 3 Complex Ions tetrahydroxyaluminate(III) [Al(OH)4] Step one – add Na. OH Al 3+ + 3](https://slidetodoc.com/presentation_image_h2/42081e38f11d429f402b8479c236b636/image-28.jpg)
Complex Ions tetrahydroxyaluminate(III) [Al(OH)4] Step one – add Na. OH Al 3+ + 3 OH Al(OH)3 Step two – add excess Na. OH Al(OH)3 + OH [Al(OH)4] OH- OHOH- Al 3+ 2013 Chemistry NCEA L 2 2. 1 Identify Ions GZ OH- + OH- Al 3+ OH- OHSJ Gaze
![Complex Ions tetraamminezincII ZnNH 342 tetraamminecopperII CuNH 342 NH 3 H 2 O Complex Ions tetraamminezinc(II) [Zn(NH 3)4]2+ tetraamminecopper(II) [Cu(NH 3)4]2+ NH 3 + H 2 O](https://slidetodoc.com/presentation_image_h2/42081e38f11d429f402b8479c236b636/image-29.jpg)
Complex Ions tetraamminezinc(II) [Zn(NH 3)4]2+ tetraamminecopper(II) [Cu(NH 3)4]2+ NH 3 + H 2 O NH 4+ + OH- Step one – add 2 drops NH 3 Zn 2+ + 2 OH Zn(OH)2 Step two – add excess NH 3 Zn(OH)2 OH- + 4 NH 3 Zn 2+ OH- [Zn(NH 3)4]2+ + + NH 3 2013 Chemistry NCEA L 2 2. 1 Identify Ions GZ OH- NH 3 OH- 2 OH - Zn 2+ NH 3 SJ Gaze
![Complex Ions diamminesilverI AgNH 32 NH 3 H 2 O NH 4 Complex Ions diamminesilver(I) [Ag(NH 3)2]+ NH 3 + H 2 O NH 4+ +](https://slidetodoc.com/presentation_image_h2/42081e38f11d429f402b8479c236b636/image-30.jpg)
Complex Ions diamminesilver(I) [Ag(NH 3)2]+ NH 3 + H 2 O NH 4+ + OH- Step one – add 2 drops NH 3 Ag+ + OH Ag. OH Step two – add excess NH 3 Ag. OH + 2 NH 3 [Ag(NH 3)2]+ + OH OH- Ag+ OH- + NH 3 Ag+ NH 3 2013 Chemistry NCEA L 2 2. 1 Identify Ions GZ SJ Gaze

Complex Ions iron(III) thiocyanate Fe. SCN 2+ Step one – add KSCN Fe 3+ + SCN Fe. SCN 2+ K+ K+ Fe 3+ 2013 Chemistry NCEA L 2 2. 1 Identify Ions GZ Fe 3+ + SCN- SJ Gaze

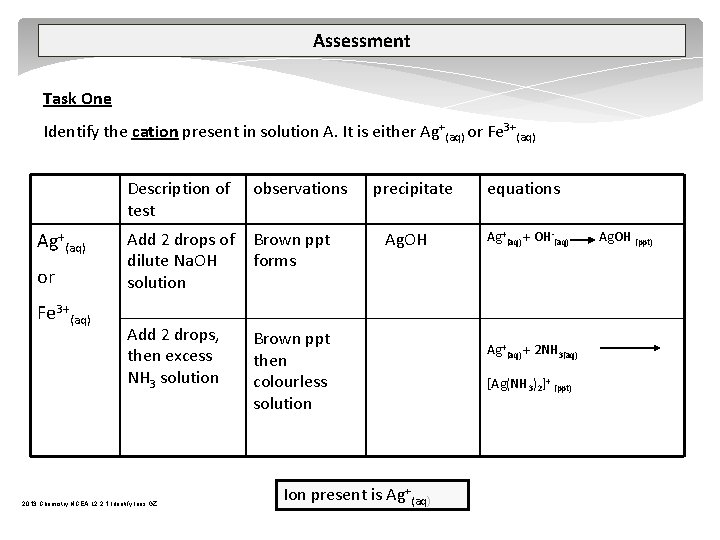
Assessment Task One Identify the cation present in solution A. It is either Ag+(aq) or Fe 3+(aq) Description of test observations Add 2 drops of dilute Na. OH solution Brown ppt forms Add 2 drops, then excess NH 3 solution Brown ppt then colourless solution 2013 Chemistry NCEA L 2 2. 1 Identify Ions GZ precipitate Ag. OH Ion present is Ag+(aq) equations Ag+(aq) + OH (aq) Ag+(aq) + 2 NH 3(aq) [Ag(NH 3)2]+ (ppt) Ag. OH (ppt)

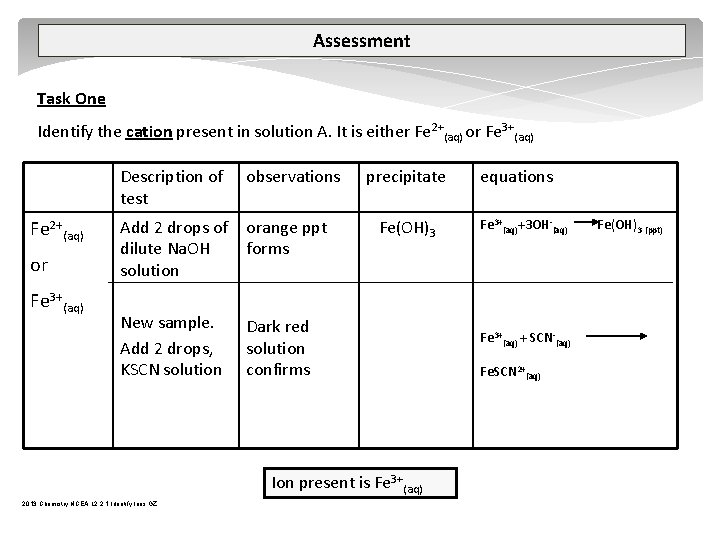
Assessment Task One Identify the cation present in solution A. It is either Fe 2+(aq) or Fe 3+(aq) Description of test observations Add 2 drops of dilute Na. OH solution orange ppt forms New sample. Add 2 drops, KSCN solution Dark red solution confirms precipitate Fe(OH)3 Ion present is Fe 3+(aq) 2013 Chemistry NCEA L 2 2. 1 Identify Ions GZ equations Fe 3+(aq)+3 OH (aq) Fe 3+(aq) + SCN (aq) Fe. SCN 2+(aq) Fe(OH)3 (ppt)

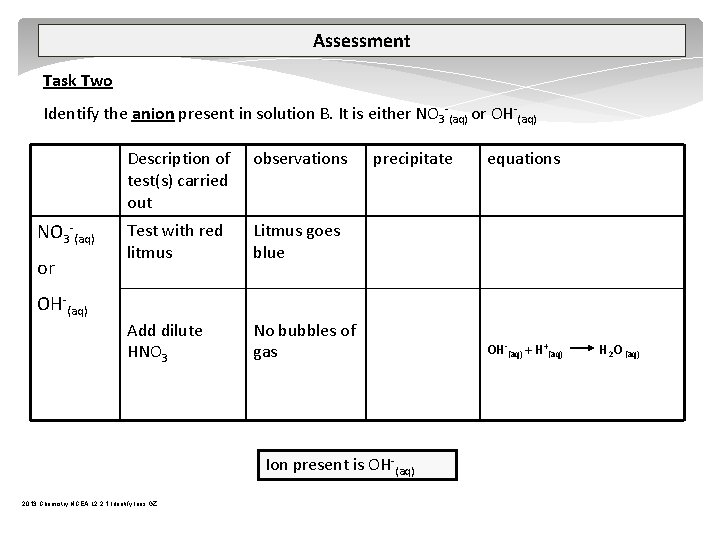
Assessment Task Two Identify the anion present in solution B. It is either NO 3 (aq) or OH (aq) Description of test(s) carried out observations Test with red litmus Litmus goes blue Add dilute HNO 3 No bubbles of gas precipitate Ion present is OH (aq) 2013 Chemistry NCEA L 2 2. 1 Identify Ions GZ equations OH (aq) + H+(aq) H 2 O (aq)

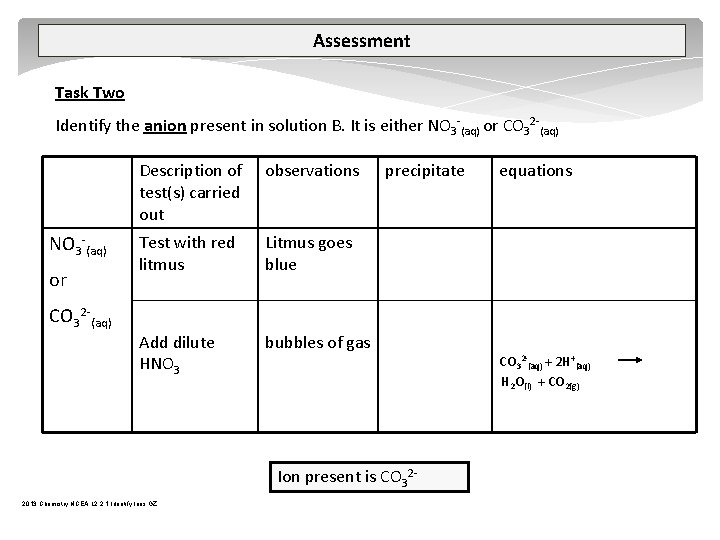
Assessment Task Two Identify the anion present in solution B. It is either NO 3 (aq) or CO 32 (aq) Description of test(s) carried out observations Test with red litmus Litmus goes blue Add dilute HNO 3 bubbles of gas precipitate Ion present is CO 32 2013 Chemistry NCEA L 2 2. 1 Identify Ions GZ equations CO 32 (aq) + 2 H+(aq) H 2 O(l) + CO 2(g)

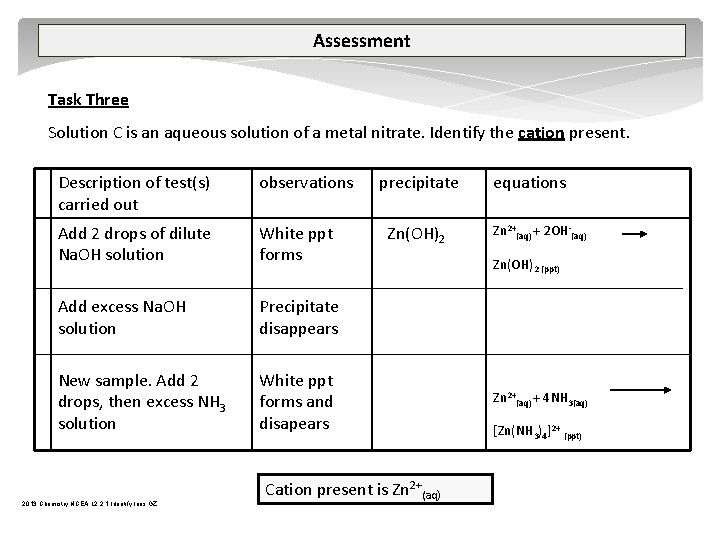
Assessment Task Three Solution C is an aqueous solution of a metal nitrate. Identify the cation present. Description of test(s) carried out observations Add 2 drops of dilute Na. OH solution White ppt forms Add excess Na. OH solution Precipitate disappears New sample. Add 2 drops, then excess NH 3 solution White ppt forms and disapears 2013 Chemistry NCEA L 2 2. 1 Identify Ions GZ precipitate Zn(OH)2 Cation present is Zn 2+(aq) equations Zn 2+(aq) + 2 OH (aq) Zn(OH)2 (ppt) Zn 2+(aq) + 4 NH 3(aq) [Zn(NH 3)4]2+ (ppt)

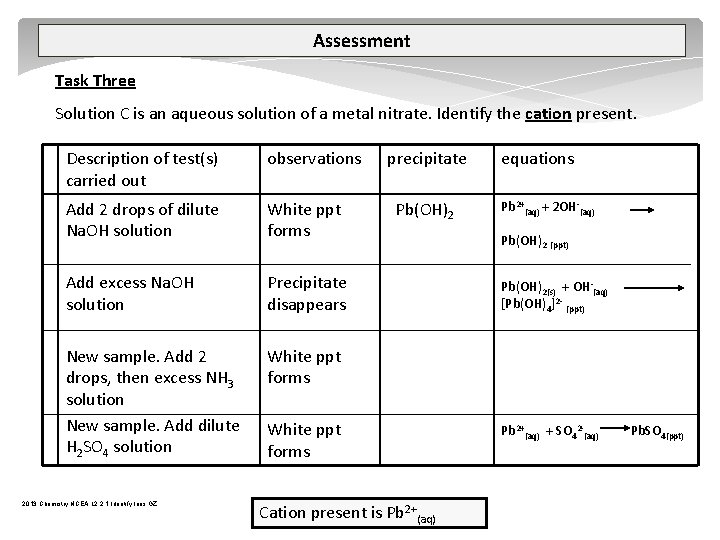
Assessment Task Three Solution C is an aqueous solution of a metal nitrate. Identify the cation present. Description of test(s) carried out observations Add 2 drops of dilute Na. OH solution White ppt forms Add excess Na. OH solution Precipitate disappears New sample. Add 2 drops, then excess NH 3 solution New sample. Add dilute H 2 SO 4 solution White ppt forms 2013 Chemistry NCEA L 2 2. 1 Identify Ions GZ precipitate Pb(OH)2 White ppt forms Cation present is Pb 2+(aq) equations Pb 2+(aq) + 2 OH (aq) Pb(OH)2 (ppt) Pb(OH)2(s) + OH (aq) [Pb(OH)4]2 (ppt) Pb 2+(aq) + SO 42 (aq) Pb. SO 4(ppt)

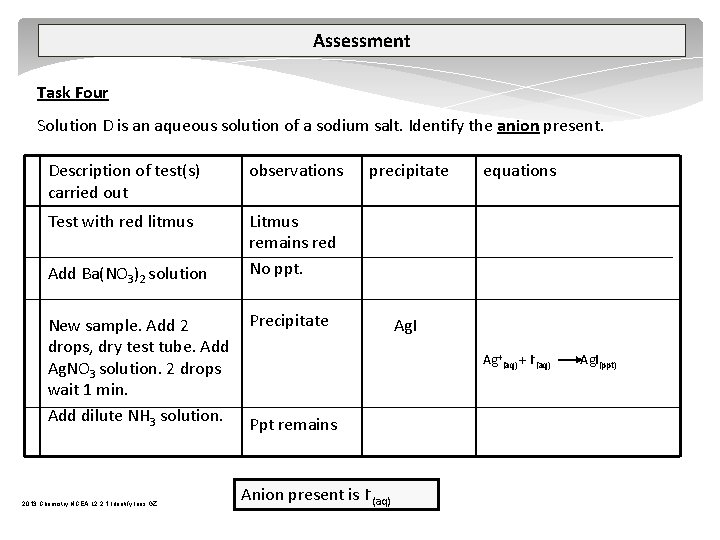
Assessment Task Four Solution D is an aqueous solution of a sodium salt. Identify the anion present. Description of test(s) carried out observations Test with red litmus Litmus remains red No ppt. Add Ba(NO 3)2 solution New sample. Add 2 drops, dry test tube. Add Ag. NO 3 solution. 2 drops wait 1 min. Add dilute NH 3 solution. 2013 Chemistry NCEA L 2 2. 1 Identify Ions GZ precipitate Precipitate equations Ag. I Ag+(aq) + I (aq) Ppt remains Anion present is I (aq) Ag. I(ppt)

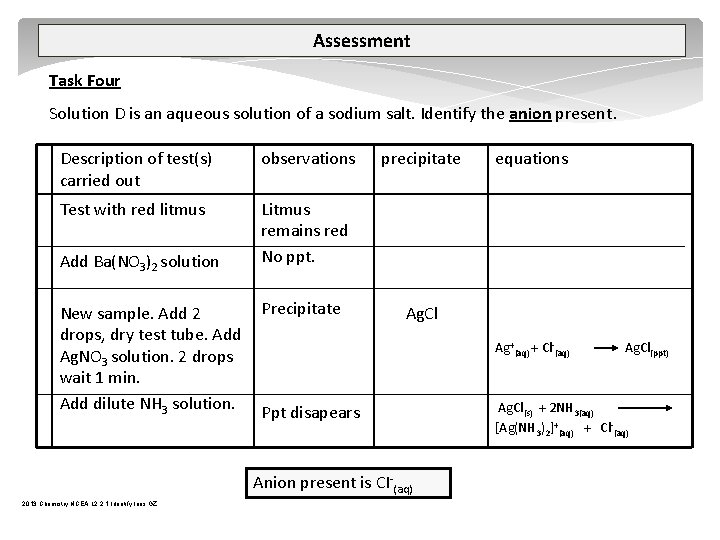
Assessment Task Four Solution D is an aqueous solution of a sodium salt. Identify the anion present. Description of test(s) carried out observations Test with red litmus Litmus remains red No ppt. Add Ba(NO 3)2 solution New sample. Add 2 drops, dry test tube. Add Ag. NO 3 solution. 2 drops wait 1 min. Add dilute NH 3 solution. Precipitate precipitate Ag. Cl Ag+(aq) + Cl (aq) Ppt disapears Anion present is CI (aq) 2013 Chemistry NCEA L 2 2. 1 Identify Ions GZ equations Ag. Cl(ppt) Ag. Cl(s) + 2 NH 3(aq) [Ag(NH 3)2]+(aq) + Cl (aq)

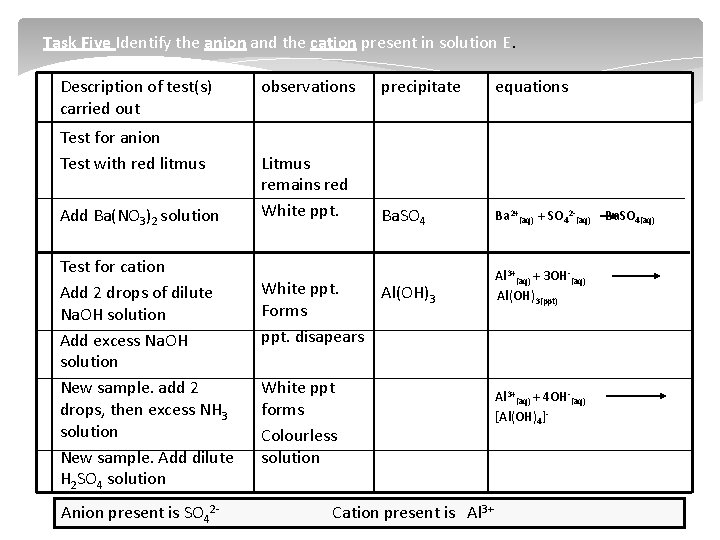
Task Five Identify the anion and the cation present in solution E. Description of test(s) carried out Test for anion Test with red litmus Add Ba(NO 3)2 solution Test for cation Add 2 drops of dilute Na. OH solution Add excess Na. OH solution New sample. add 2 drops, then excess NH 3 solution New sample. Add dilute H 2 SO 4 solution Anion present is SO 42 observations precipitate equations Litmus remains red White ppt. Ba. SO 4 Ba 2+(aq) + SO 42 (aq) White ppt. Al(OH)3 Forms ppt. disapears White ppt forms Colourless solution Cation present is Al 3+(aq) + 3 OH (aq) Al(OH)3(ppt) Al 3+(aq) + 4 OH (aq) [Al(OH)4] Ba. SO 4(aq)

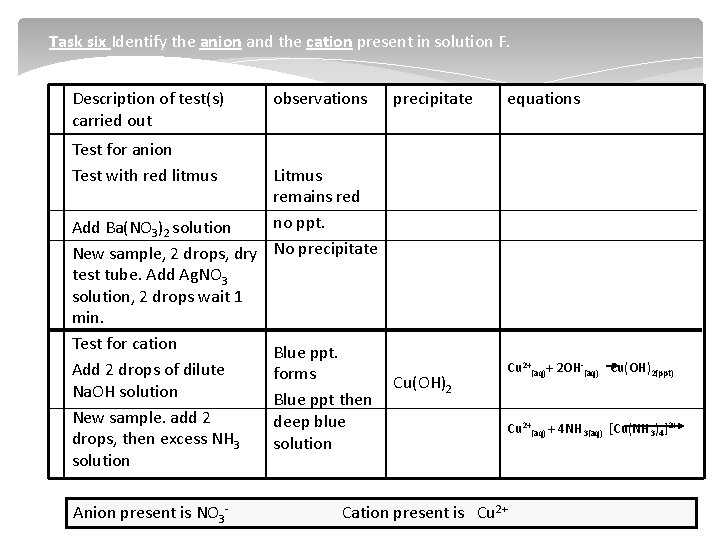
Task six Identify the anion and the cation present in solution F. Description of test(s) carried out observations precipitate equations Test for anion Test with red litmus Litmus remains red no ppt. Add Ba(NO 3)2 solution New sample, 2 drops, dry No precipitate test tube. Add Ag. NO 3 solution, 2 drops wait 1 min. Test for cation Blue ppt. Add 2 drops of dilute forms Cu(OH)2 Na. OH solution Blue ppt then New sample. add 2 deep blue drops, then excess NH 3 solution Anion present is NO 3 Cu 2+(aq)+ 2 OH (aq) Cu(OH)2(ppt) Cu 2+(aq) + 4 NH 3(aq) [Cu(NH 3)4]2+ Cation present is Cu 2+