Venous Thrombosis in Pregnancy Dr R sami Pulmonologist

Venous Thrombosis in Pregnancy Dr. R. sami Pulmonologist IUMS
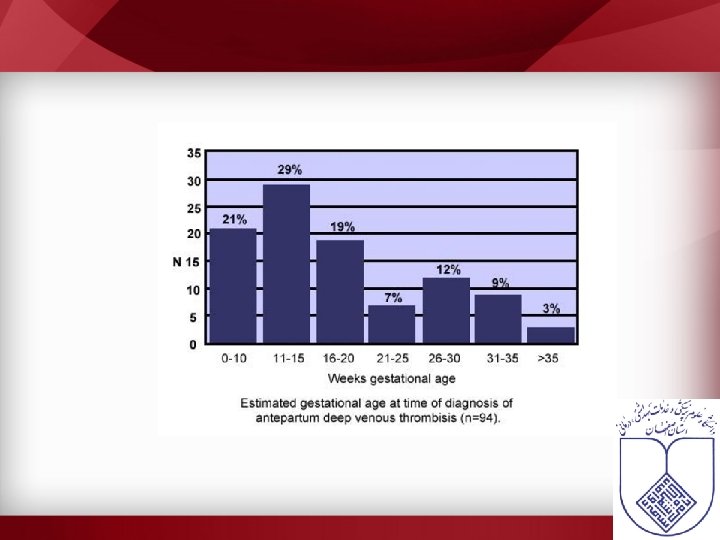
VTE in pregnancy • Pregnant women are at an increased risk for venous thromboembolic disease (VTE) – 1 in 1000 pregnancies – 2 -4 fold increase compared to non-pregnant state – Cesarian delivery > vaginal delivery – 2/3 of DVT occur antepartum – 43 -60% of PE occur 4 -6 weeks after delivery • PE is the major non-obstetric cause of maternal mortality – 2/100 000 pregnancies

Why is the risk greater in pregnancy? • Pathopsysiology – Increased venous capacity (estrogen) – Increased plasma volume – Compression of IVC – Increased levels of coagulation factors (fibrinogen, factor VII) – Decreased levels of natural anticoagulants (protein S) – Acquired protein C resistance
Independent risk factors • • • Bed rest Multiparity Advanced maternal age (>35 yo) Overweight Personal or family history of VTE Preeclampsia

DVT

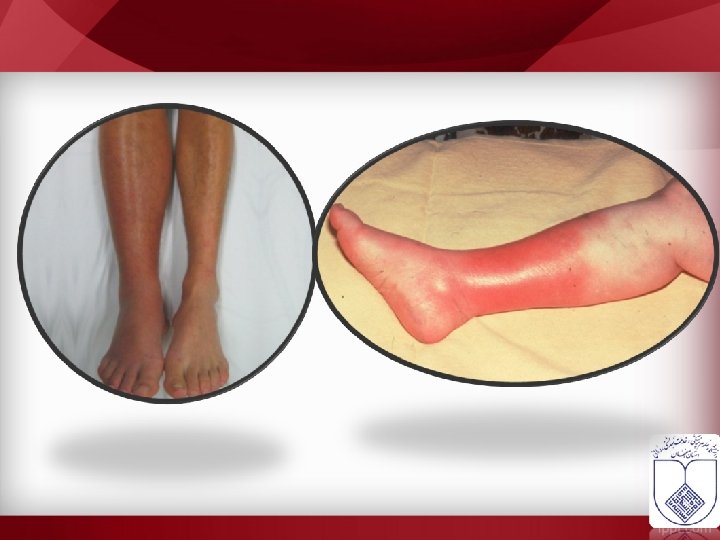
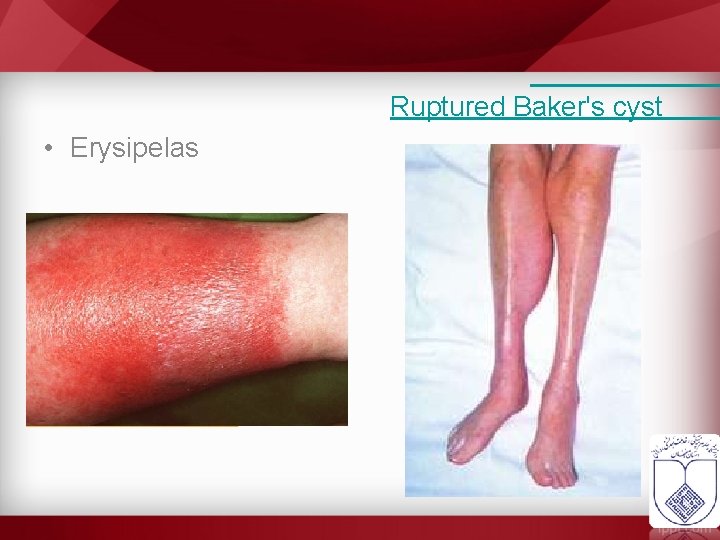
Ruptured Baker's cyst • Erysipelas
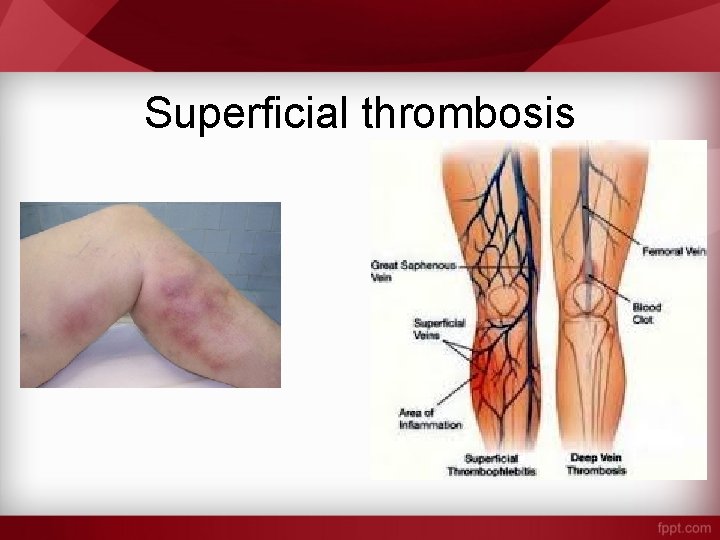
Superficial thrombosis
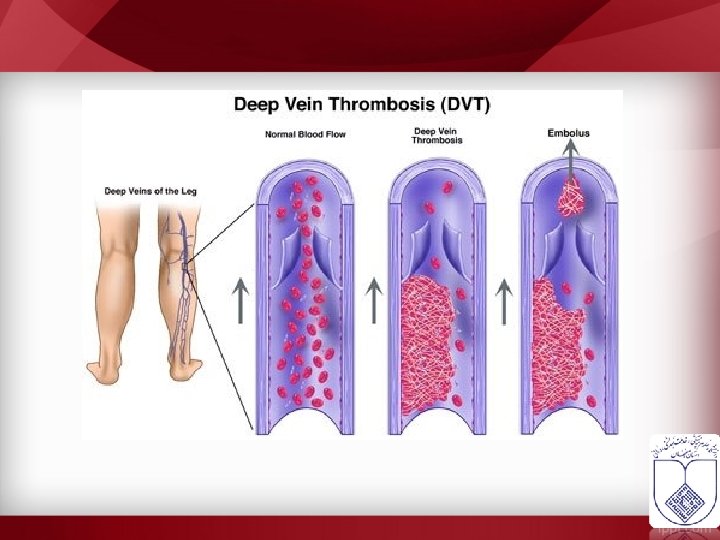

DVT – VTE Risk Factors • • • Malignancy Surgery Trauma Pregnancy Oral contraceptives or hormonal therapy • Immobilization • Inherited thrombophillia • Presence of venous catheter • Congestive failure • Antiphospholipid antibody syndrome • Hyperviscosity • Nephrotic syndrome • Inflammatory bowel disease
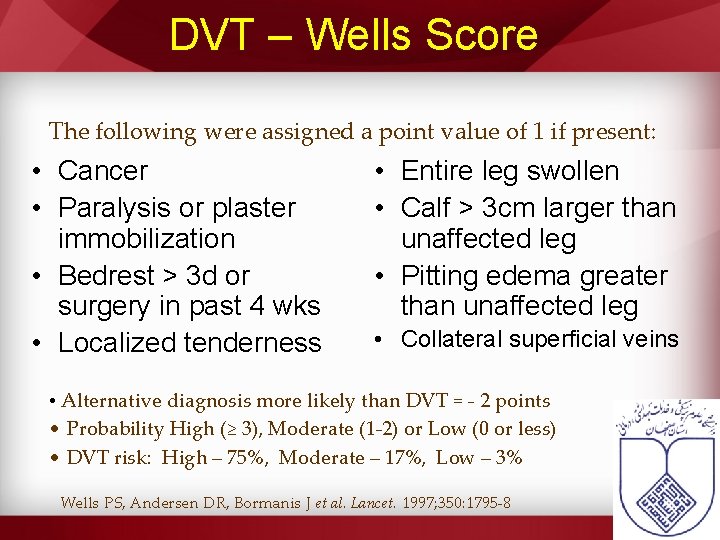
DVT – Wells Score The following were assigned a point value of 1 if present: • Cancer • Paralysis or plaster immobilization • Bedrest > 3 d or surgery in past 4 wks • Localized tenderness • Entire leg swollen • Calf > 3 cm larger than unaffected leg • Pitting edema greater than unaffected leg • Collateral superficial veins • Alternative diagnosis more likely than DVT = - 2 points • Probability High (≥ 3), Moderate (1 -2) or Low (0 or less) • DVT risk: High – 75%, Moderate – 17%, Low – 3% Wells PS, Andersen DR, Bormanis J et al. Lancet. 1997; 350: 1795 -8

Challenge in diagnosis

DVT – D-Dimer • Fibrin degradation product elevated in active thrombosis • Negative …. . Positive…. • Preferred test – Quantitative Rapid ELISA – sensitivity 96/95% for DVT/PE – Other methods include latex agglutination and RBC agglutination (Simpli. RED) Stein PD, Hull RD, Patel KC, et al. Ann Int Med. 2004; 140(8): 589 -602
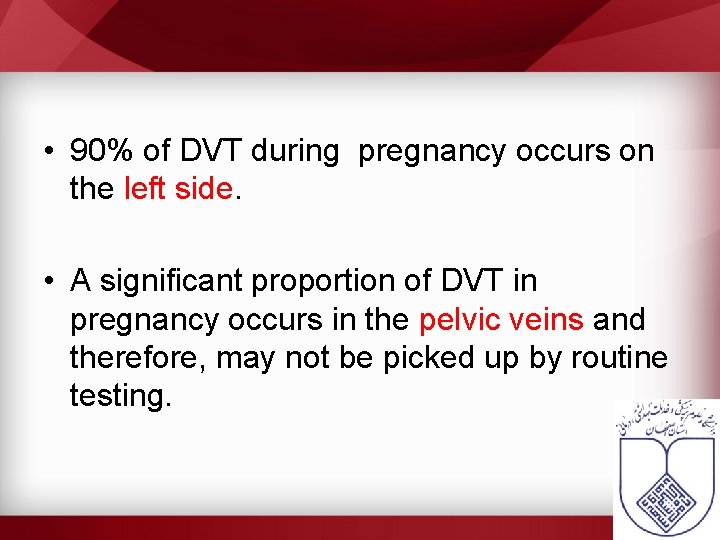
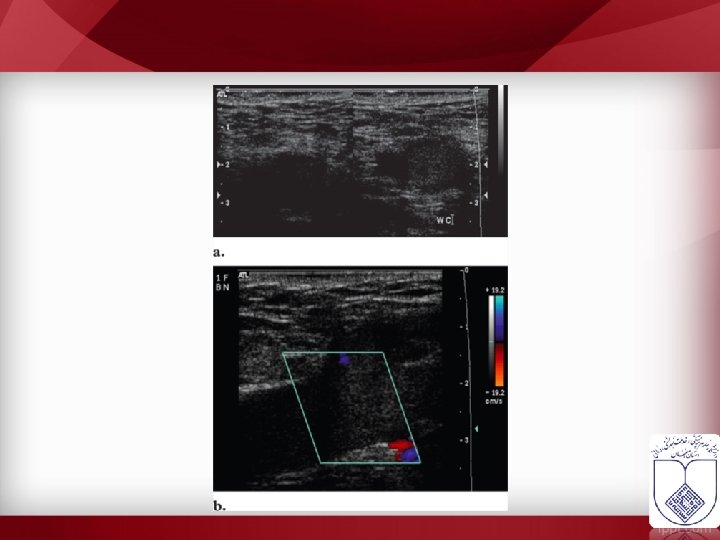
• 90% of DVT during pregnancy occurs on the left side. • A significant proportion of DVT in pregnancy occurs in the pelvic veins and therefore, may not be picked up by routine testing.

• Calf • Proximal • Ultrasonography ? ? • Approach ? ?

PTE

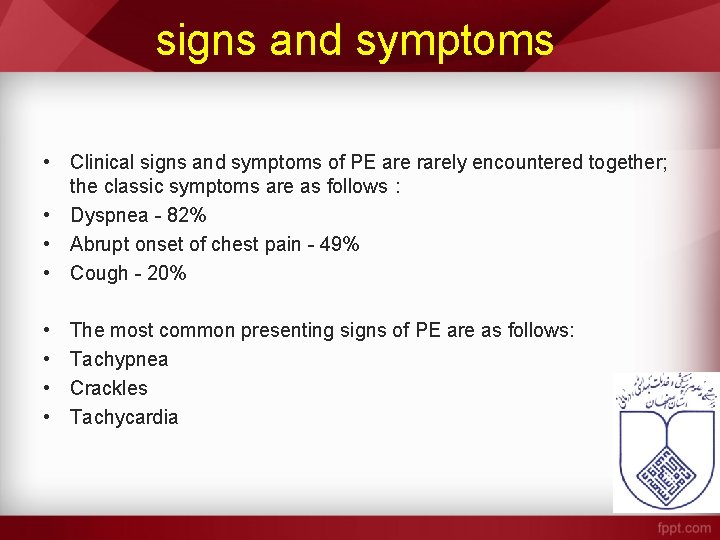
signs and symptoms • Clinical signs and symptoms of PE are rarely encountered together; the classic symptoms are as follows : • Dyspnea - 82% • Abrupt onset of chest pain - 49% • Cough - 20% • • The most common presenting signs of PE are as follows: Tachypnea Crackles Tachycardia
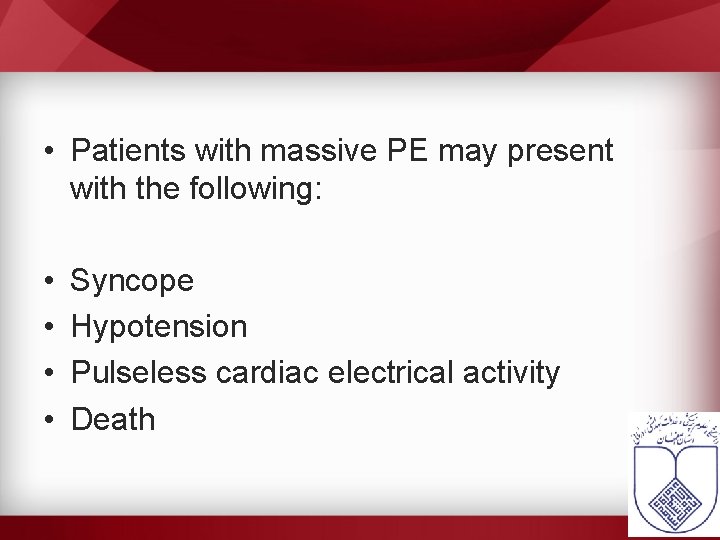
• Patients with massive PE may present with the following: • • Syncope Hypotension Pulseless cardiac electrical activity Death
LEFt • L- Symptoms in the left lower extremity • E-Edema: Mid-calf circumference difference of ≥ 2 cm • Ft- First trimester presentation No finding 0 1 finding 16% 2 or 3 findings 58%.
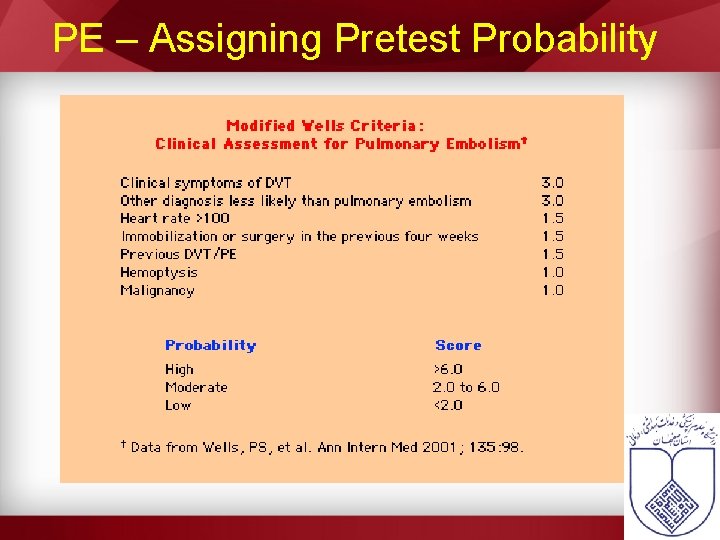
PE – Assigning Pretest Probability

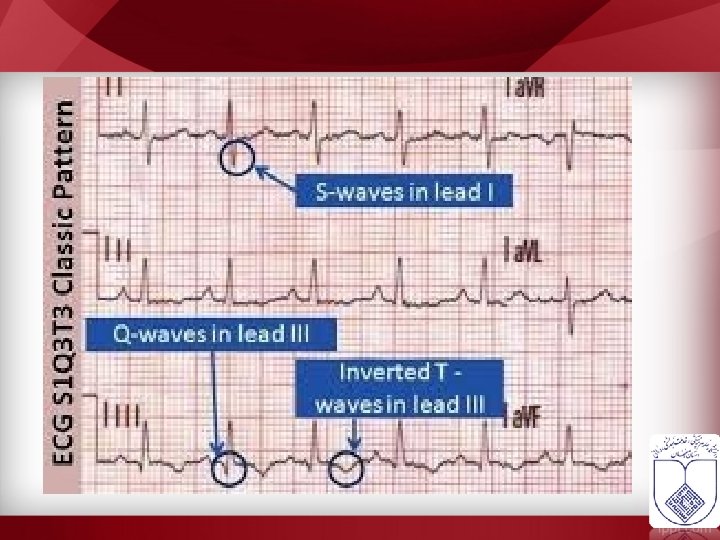
Para clinic • If DVT or PE is suspected, the patient should begin anticoagulation treatment until further investigation excludes VTE. • • • ECG ABG CXR CT Scan
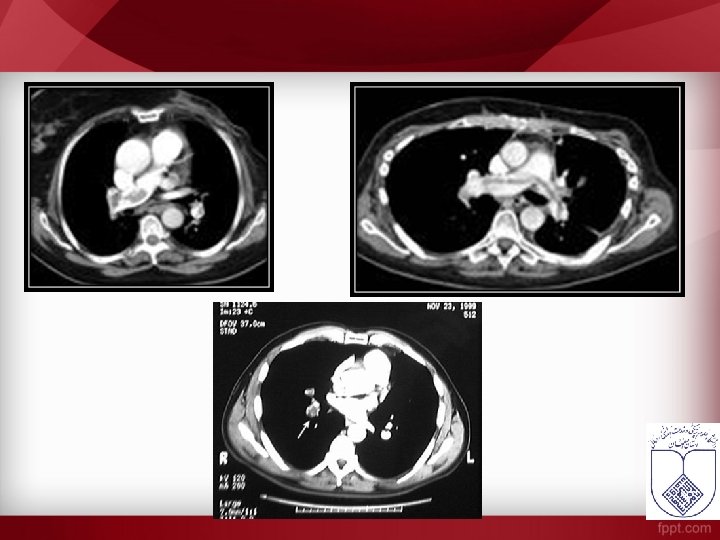
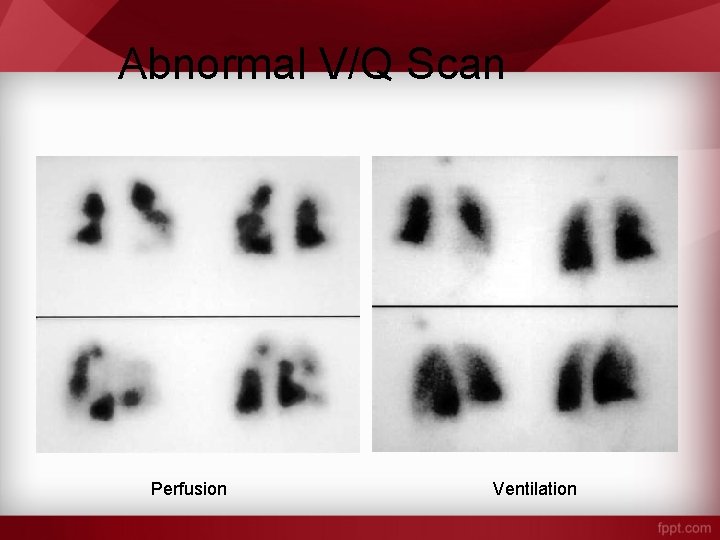
Abnormal V/Q Scan Perfusion Ventilation

fetal risks from radiation doses of less than 50 m. Gy are negligible doses of 100 m. Gy and more result in a combined increased risk of organ malformation and the development of childhood cancer of only about 1%

even a combination of imagings( chest radiography, lung scintigraphy, CT pulmonary angiography, and traditional pulmonary angiography )exposesthe fetus to around 1. 5 m. Gy of radiation(below the accepted limit of 50 m. Gy)
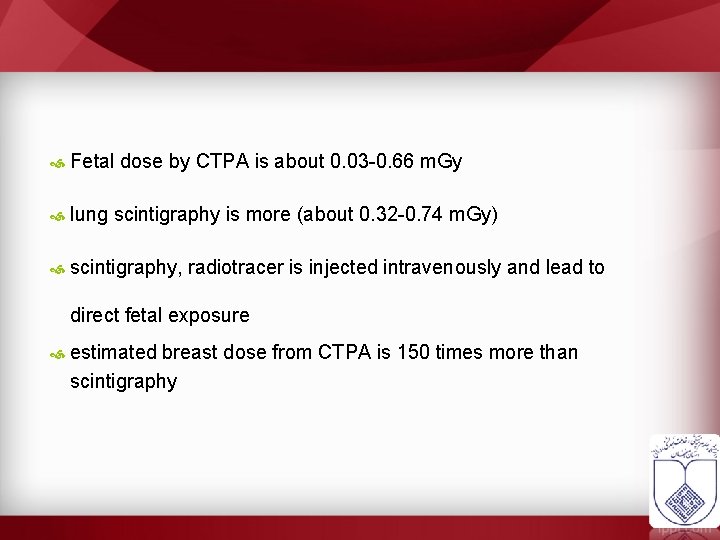
Fetal dose by CTPA is about 0. 03 -0. 66 m. Gy lung scintigraphy is more (about 0. 32 -0. 74 m. Gy) scintigraphy, radiotracer is injected intravenously and lead to direct fetal exposure estimated breast dose from CTPA is 150 times more than scintigraphy
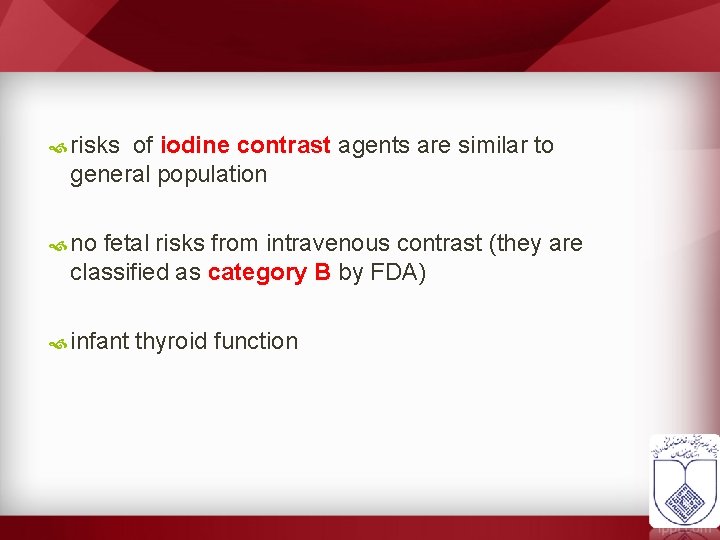
risks of iodine contrast agents are similar to general population no fetal risks from intravenous contrast (they are classified as category B by FDA) infant thyroid function
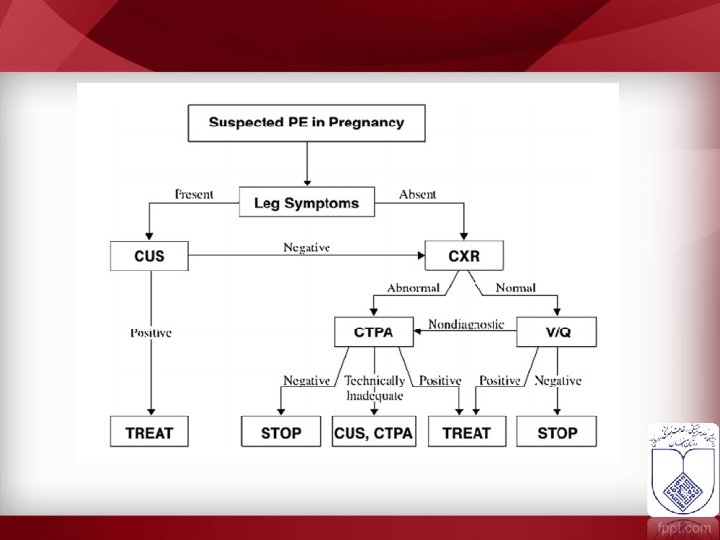

Treatment


- Slides: 38