Variant Curation Evidence Summaries Variant Curation Evidence Summaries







- Slides: 11

Variant Curation Evidence Summaries

Variant Curation Evidence Summaries ● FDA requirement for expert panel classifications in Clin. Var (also required for pilot variants for SVI approval) Direct quote from Clin. Gen’s FDA decision summary: Each VCEP is responsible for developing variant evidence summaries that promote understanding of the evidence used to support the assertion and enable external review. The evidence summaries are made in clear and understandable language and include the pathogenicity classification, the evidence supporting the application of each criterion, and the reason for applying each criterion. These statements include, where applicable, reference to the external data sources used in the curation and classification. These evidence summaries are provided in the summary evidence tab in Clin. Var. They are also accessible within Clin. Gen’s evidence repository, which is publicly accessible to all users.

Variant Curation Evidence Summaries ● Often helpful to have a template for ease and consistency (a general template is in the works now) ● Detail the evidence used to classify a variant ○ ○ ○ ACMG codes applied Internal data from members of VCEP Data from the literature ● Saved in the VCI, included in Clin. Var submission ● Can be accessed by the community in a variety of different ways

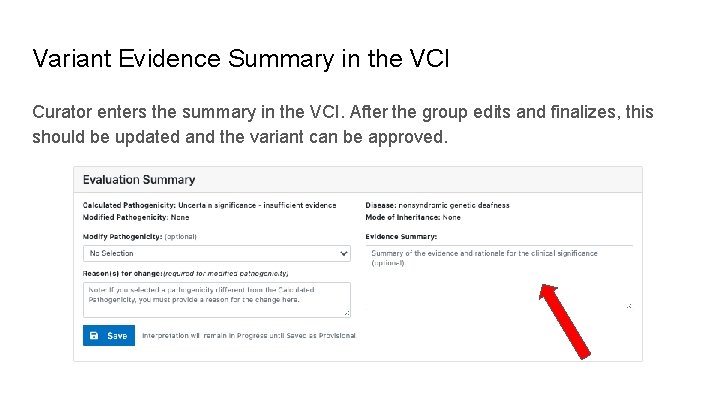
Variant Evidence Summary in the VCI Curator enters the summary in the VCI. After the group edits and finalizes, this should be updated and the variant can be approved.

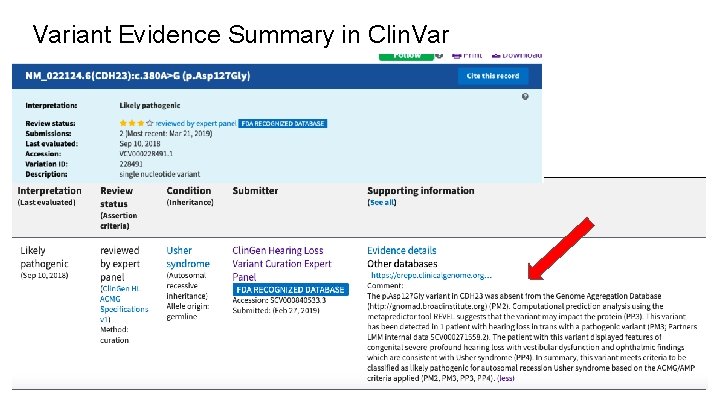
Variant Evidence Summary in Clin. Var

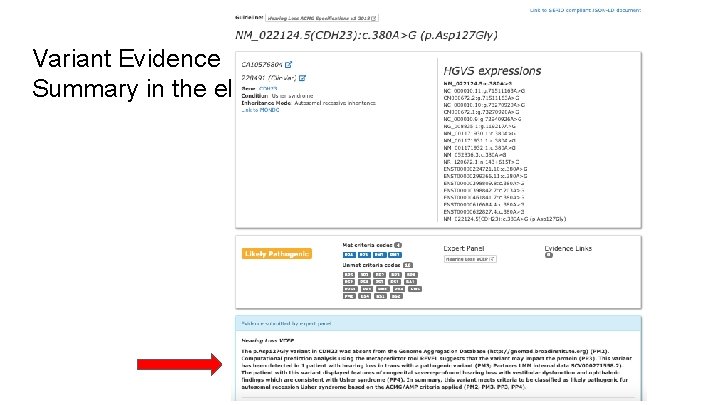
Variant Evidence Summary in the e. Repo

Suggestions for editing variant evidence summaries ● Curators/coordinators provide a google doc to be edited in real time during a variant presentation on a VCEP call ● Experts can be assigned (usually two per summary) to wordsmith the summary afterward ● The VCEP chairs can either approve them offline or in real time on a chairs’ call **The variant classification should either be approved by the group or the core group of approvers per the VCEP application. These are just suggestions for finalizing the evidence summary.

Examples: Hearing Loss VCEP The allele frequency of the c. 401 T>A (p. Ile 134 Asn) variant in the MYO 7 A gene is 0. 0062% (7/112888) of European (non-Finnish) chromosomes by gnom. AD, which is a low enough frequency to apply PM 2 based on the thresholds defined by the Clin. Gen Hearing Loss Expert Panel for autosomal recessive hearing loss. This variant has been detected in 5 probands with Usher syndrome. For 2 of those probands, pathogenic variants (c. 19 -1 G>A and Gly 214 Arg) were observed in trans, and in the other 3 probands, a pathogenic variant was observed with unknown phase (PM 3_Strong, PMIDs: 25468891, 26969326, Partners LMM Internal Data Clin. Var SCV 000059786. 6). The variant has been reported to segregate with hearing loss in one affected family member (PP 1, Partners LMM Internal Data Clin. Var SCV 000059786. 6). The REVEL computational prediction tool produced a score of 0. 938, which is above threshold necessary to apply PP 3. At least one patient with this variant displayed features of sensorineural hearing loss and retinitis pigmentosa, which are consistent for Usher syndrome, a condition highly specific for MYO 7 A (PP 4, Partners LMM Internal Data Clin. Var SCV 000059786. 6). In summary, this variant meets criteria to be classified as likely pathogenic for autosomal recessive Usher syndrome based on the ACMG/AMP criteria applied as specified by the Hearing Loss Expert Panel (PM 3_Strong, PM 2, PP 1, PP 3, PP 4).

Examples: RASopathy VCEP The c. 1113 T>C (p. Asp 371=) is present in 7/1113684 European alleles (MAF 2. 88 e-05, 95% CI) gnom. AD v 2. 1. 1. Sixteen apparently unaffected parental samples involved in whole exome testing were observed with this variant supporting that this variant is likely benign; however, this evidence does not meet current scoring criteria for BS 2 at this time (BS 2 not met; SCV 000515669. 4). This variant is a synonymous (silent) variant at a nucleotide that is not highly conserved and is not predicted to impact splicing (BP 7). Computational prediction tools and conservation analysis suggest that the p. Asp 371= variant does not impact the protein (BP 4). In summary, the clinical significance of the p. Asp 371= variant is likely benign. RASopathy-specific ACMG/AMP criteria applied (PMID: 29493581): BP 7, BP 4.

Example summary documents Often helpful to organize by code Hearing loss example It can also be helpful to organize by type of data per ACMG grid E. g. Population data, computational predictors, etc LMM example

Questions? Comments?