Vanadium has different oxidation states V 5 d




![• Complexes of v 4+ + Cl] Cl [VO(dipy)2 [VO(acac)2]2+Cl 2 [VO(NCS)4]2 - • Complexes of v 4+ + Cl] Cl [VO(dipy)2 [VO(acac)2]2+Cl 2 [VO(NCS)4]2 -](https://slidetodoc.com/presentation_image_h2/8cbc24a042211cc90fb1e82b784ea549/image-6.jpg)














![complexes of Mn 2+ [Mn(en)2]2+ , [Mn(EDTA)]2+ , [Mn(H 2 O)6]2+ (Mn 3+) Ø complexes of Mn 2+ [Mn(en)2]2+ , [Mn(EDTA)]2+ , [Mn(H 2 O)6]2+ (Mn 3+) Ø](https://slidetodoc.com/presentation_image_h2/8cbc24a042211cc90fb1e82b784ea549/image-21.jpg)
![complexes of Mn 3+ [Mn(acac)3]3+ , [Mn(CN)6]3 - , [Mn(C 2 O 4)3]3(Mn 4+) complexes of Mn 3+ [Mn(acac)3]3+ , [Mn(CN)6]3 - , [Mn(C 2 O 4)3]3(Mn 4+)](https://slidetodoc.com/presentation_image_h2/8cbc24a042211cc90fb1e82b784ea549/image-22.jpg)


- Slides: 24


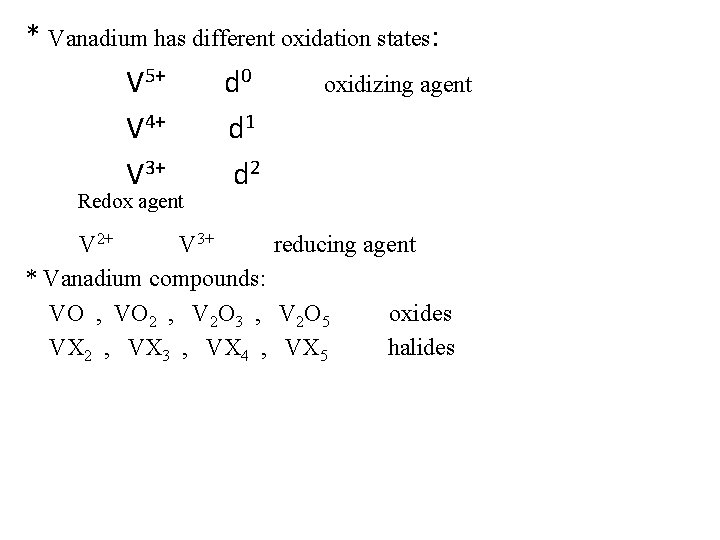
* Vanadium has different oxidation states: V 5+ d 0 oxidizing agent V 4+ d 1 V 3+ d 2 Redox agent V 2+ V 3+ reducing agent * Vanadium compounds: VO , VO 2 , V 2 O 3 , V 2 O 5 oxides VX 2 , VX 3 , VX 4 , VX 5 halides

(V 2+) V 4+ V 2+ H 2/Zn K 2 SO 4 V 2+ VSO 4. K 2 SO 4. n. H 2 O (double salt) VX 2 CN[V(CN)6] -4 (complex) V 2 O 5 H 2/Oxalic VO X 2

(V 3+) V 4+ H 2/Zn V 3+ X 2 VX 3 VX 4 Red VX 3 VOX 2 Red VOX V 2 O 5 H 2/∆ V 2 O 3 (basic oxide) HCl V 3+ [V(H 2 O)6]Cl 3 C 2 O 4 [V(C 2 O 4)3] CN [V(CN)6]3 SCN [V(SCN)6]3 -

(V 4+) V 5+ Red/H 2 V 4+ VX 4 , VOX 2 , VO 2 Halides , oxohalides , oxides VOX 3 Red VOX 2 VOCl 3 H 2 VOCl 2 § V 4+ + X 2 VX 4 VCl 4 + HF VF 4 + HCl § V 2 O 5 H 2/Oxalic VO 2 Ca. O Ca. VO 3
![Complexes of v 4 Cl Cl VOdipy2 VOacac22Cl 2 VONCS42 • Complexes of v 4+ + Cl] Cl [VO(dipy)2 [VO(acac)2]2+Cl 2 [VO(NCS)4]2 -](https://slidetodoc.com/presentation_image_h2/8cbc24a042211cc90fb1e82b784ea549/image-6.jpg)
• Complexes of v 4+ + Cl] Cl [VO(dipy)2 [VO(acac)2]2+Cl 2 [VO(NCS)4]2 -

• (V 5+) V + O 2 ∆/5000 C V 2 O 5 NH 4. VO 3 ∆ V 2 O 5 • V 2 O 5 is strong oxidizing agent V 2 O 5 H 2/∆ V 2 O 3 + VO V 2 O 5 ∆/Red. Agent VO 2 • V 2 O 5 is acidic oxide V 2 O 5 Base MVO 3 V 2 O 5 + HCl VCl 4 + Cl 2 + H 2 O V 2 O 5 + HCl VOCl 2 + H 2 O


• Group VI (Cr - Mo - W) • Electronic configuration ns 2(n-1)d 4 • Oxidation states of Cr Cr 6+ (d 0) strong oxidizing agent 5+ (d 1) self redox 4+ (d 2) 3+ (d 3) 2+ (d 4) reducing agent

v. Cr can be extracted from chromite ore by two different methods: • Fe. Cr 2 O 4 C/ ∆ Fe + 2 Cr 0 + 4 CO chromite Fe. O + Cr 2 O 3 • Fe. Cr 2 O 4 Na 2 Cr 2 O 7 + Cr 0 O 2/Na. OH Al 2 O 3 C/Red Al/ ∆ Cr 2 O 3

• (Cr 2+) compounds: Ø Cr. Hg O 2 Cr. O O 2 Cr 2 O 3 Cr 2+ + Na. OH Cr(OH)2 Cr. O + H 2 SO 4 Cr. SO 4 Ø Cr Cr. Cl 3 Cr. Cl 2 HCl H 2 N 2 H 4 Cr. Cl 2 [Cr(N 2 H 4)3]Cl 2

• (Cr 3+) compounds: 1) Cr O 2/ ∆ 2) M 2 O 3 OH-/base 3) Cr + Cl 2 Cr. Cl 3 H 2 NH 3 THF H 2 S ∆ Cr 2 O 3 Na. Cr. O 2 Na 2 Cr. O 4 H+ Na 2 Cr 2 O 7 Cr. Cl 3 Cr. Cl 2 [Cr(NH 3)6]X 3 Cr. Cl 3. 3 THF Cr 2 S 3

• (Cr 4+) compounds: • Cr. Cl 3 Cl 2 Cr. Cl 4 Ca. O • Cr. O 2 Sr. O Ba. O Ca. Cr. O 4 Sr 3 Cr. O 5 Ba 3 Cr. O 5

• (Cr 5+) compounds: • Cr + F 2 ∆/P Cr. F 5 • Cr. O 3 + BF 3 • Cr. O 2 Cl 2 + BCl 3 Cr. OF 3 oxofluoride Cr. OCl 3 oxochloride

• (Cr 6+) compunds: HCl Ø Cr. O + O 2 Ø Cr + HNO 3 Cr 2 O 3 Cr. O 2 Cl 2 Na. OH H 2 O Na. Cr. O 4 Na 2 Cr. O 4 O 2 Na 2 Cr 2 O 7 Ø Fe. Cl 2 O 4 Ø Cr. O 4 2 - H+ Na 2 Cr 2 O 72 - strong oxidizing agent O 2/Na. OH H+/acid Na 2 Cr. O 4

Cr 3+ + Sn 4+ Ø Cr 2 O 7 H+/Fe 2+ Fe 3+ + Cr 3+ H 2 O Cr(OH)3 + OHØ K 2 Cr 2 O 7 + KCl conc H SO Cr. O 2 Cl 2 strong oxidizing agent H+/Sn 2+ 2 Ø Cr. O 2 Cl 2 Ø Cr + F 2 4 H 2 Cr. O 4 900 C/P 350 Cr. F 6 At r. t Cr. OF 4 Ø Cr. O 2 Cl 2 + F 2 Cr. O 2 F 2 H 2 O 0


v. Group VII (Mn - TC - Re) Ø Electronic configuration n. S 2(n-1)d 5 Ø Oxidation states of Mn : o (2+) d 5 strong reducing agent , more stable , it has half filled d o (3+) d 4 strong oxidizing agent , unstable and converted easily to 2+ o (4+) d 3 Mn. O 2 is the most important cpd o (6+) Mn exhibit as Mn. O 42 o (7+) Mn exhibit as Mn. O 4Ø Mn 5+ not exist

Ø Mn 2+ Mn. O Mn 3+ Mn 2 O Mn 4+ Mn 6+ Mn 7+ Mn. O 2 Mn. O 42 - Mn. O 4 - Ø Mn can be extracted from pyrolosite ore (Mn. O 2) : § 3 Mn. O 2 ∆/O 2 Mn 3 O 4 + O 2 Al/ ∆ Al 2 O 3 + Mn § Mn very inert at room temp. but at high temp. react with non metals. Cl 2 Mn. Cl 2 F 2 Mn. F 2 + Mn. F 3 Mn O 2 Mn 3 O 4 C Mn 2 C

(Mn 2+) reducing agent. OX/H+ Mn 2+ OX/OHPb. O/H+ HCl Mn 2+ H 2 SO 4 OHAC Mn. CO 3 ∆ Mn 4+ Mn 3+ Mn 7+ H 2 O Mn + Mn. O 2 Mn. Cl 2 Mn. SO 4 Mn(OH)2 Xss OH- Mn(OH)3 Mn(Ac)2 Mn. O + CO 2
![complexes of Mn 2 Mnen22 MnEDTA2 MnH 2 O62 Mn 3 Ø complexes of Mn 2+ [Mn(en)2]2+ , [Mn(EDTA)]2+ , [Mn(H 2 O)6]2+ (Mn 3+) Ø](https://slidetodoc.com/presentation_image_h2/8cbc24a042211cc90fb1e82b784ea549/image-21.jpg)
complexes of Mn 2+ [Mn(en)2]2+ , [Mn(EDTA)]2+ , [Mn(H 2 O)6]2+ (Mn 3+) Ø Mn. O 2 Ø Mn. I 2 + F 2 ∆/9000 C Mn 2 O 3 Mn. F 3 H 2 O/KF HF Mn 2 O 3 Ø Mn 3+ H 2 O Mn 2+ + Mn 4+ KMn. F 4 K 2 Mn. F 3
![complexes of Mn 3 Mnacac33 MnCN63 MnC 2 O 433Mn 4 complexes of Mn 3+ [Mn(acac)3]3+ , [Mn(CN)6]3 - , [Mn(C 2 O 4)3]3(Mn 4+)](https://slidetodoc.com/presentation_image_h2/8cbc24a042211cc90fb1e82b784ea549/image-22.jpg)
complexes of Mn 3+ [Mn(acac)3]3+ , [Mn(CN)6]3 - , [Mn(C 2 O 4)3]3(Mn 4+) Ø Mn 2+ OX/H+ Mn 3+ H 2 O Mn 2+ + Mn 4+ Fe 2+/Sn 2+ Mn 4+ KOH K 2 Mn. O 3 O 2 K 2 Mn. O 4 H+ KMn. O 4 Mn. Cl 2 + Cl 2 OX/OH- Ø Mn 7+ Ø Mn. O 2 HCl Mn. Cl 4 Mn 4+

(Mn 6+) Ø Mn. O 2 Ø Mn. O 42 - K 2 Mn. O 3 Mn. O 4 - K 2 Mn. O 4 (Mn 7+) Ø Mn 2+ Ø Mn. O 2 K 2 Mn. O 3 Mn 7+ K 2 Mn. O 4 KMn. O 4

(Mn. O 4)- Mn 2+ Mn 3+ Mn 4+ + OHMn 4+ + H 2 O Mn 6+ q Mn 7+ strong oxidizing agent in different PH