Validation of the Combimatrix Customarray 12 K Arrays
Validation of the Combimatrix Customarray 12 K Arrays for Gene Expression Studies on Geobacter sulfurreducens Brad Postier, A. Liu, R. Di. Donato K. Nevin, D. Lovley, B. Methe´
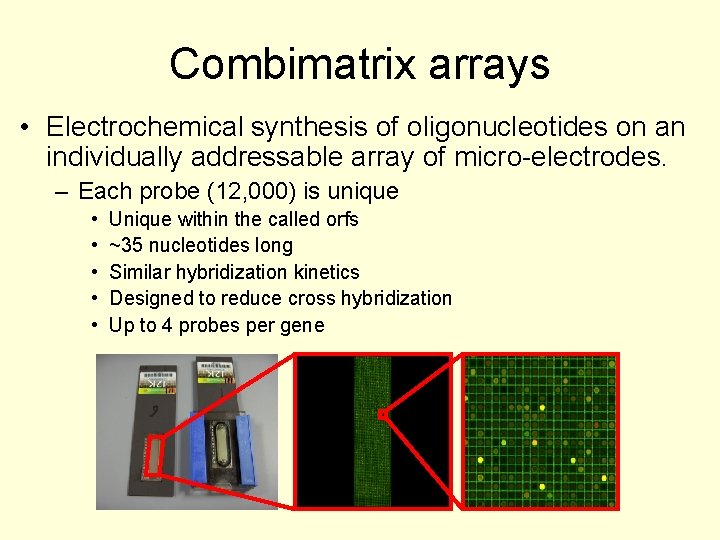
Combimatrix arrays • Electrochemical synthesis of oligonucleotides on an individually addressable array of micro-electrodes. – Each probe (12, 000) is unique • • • Unique within the called orfs ~35 nucleotides long Similar hybridization kinetics Designed to reduce cross hybridization Up to 4 probes per gene
Amplicon Arrays • PCR amplicons representing whole open reading frames from the genome of Geobacter sulfurreducens were spotted (6 times each) onto glass slides for microarray analysis • Amplicons are double stranded when spotted, then denatured after fixing to the slide – Both strands are available for hybridization
Growth conditions • Cultures of Geobacter sulfurreducens were grown in chemostats (in triplicate) to steady state conditions in freshwater media with fumarate under acetate limiting conditions in the presence or absence of ammonium chloride • The cultures were harvested for RNA extraction using Dawn’s phenol: isoamyl alcohol-chloroform protocol
Three methods were used to assay gene expression with Combimatrix arrays • Fluorescently labeled RNA • Fluorescently labeled amplified RNA(a. RNA) • Electrasesense detection of a. RNA • All RNA/a. RNA used on oligonucleotide arrays originated from the same source
Fluorescently detected arrays • 10 ug labeled RNA/sample/hybridization – Amplification was performed using the Message. Amp II for bacteria kit according to the manufacturer’s suggested protocol (Ambion). – RNA/a. RNA was labeled with the Micromax ASAP direct chemical labeling reagents according to the manufacturer’s suggested protocol(Perkin Elmer/Kreatech). – RNA/a. RNA was fragmented using Ambion’s fragmentation reagent according to the provided protocol (doubled incubation time to improve fragmentation). – Hybridizations and washes were performed in the hybridization chambers according to the protocol provided by Combimatrix using a rotisserie oven. – Slides were scanned wet (under a coverslip) with a Genepix 4000 B scanner
How do we call a gene differentially expressed? • Limma Mixed model – background signal is calculated from the mean of the bottom 30% of Negative control spots +2 SD – Loess normalization – Each probe is analyzed independently – A gene is DE if at least 50% of its probes have a p-value less than 0. 05.

Electrasense detection • Label a. RNA with biotin (Kreatech kit) • Hybridize and wash • Mix with HRP/SA • Wash excess away • Expose to Hydrogen peroxide and TMB • TMB creates a circuit with the HRP and the electrode generating a current dependent on transcript abundance

Electrasense detection • Resembles Affymetrix/Nimblegen data – One color, non-competitive hybridization – Requires more hybridizations for the same amount of data • Statistical model needs development • Labeling methods need refinement • More amenable to comparison of data across experiments – Allows more pair-wise comparisons if the same array design is used. • Less expensive and less maintenance than fluorescent detection
a. RNA Array vs. q. RT-PCR R 2 = 0. 762
Combimatrix arrays provide greater confidence through improved variance and multiple independent probes Numbers represent total genes called DE(# with mean M>+0. 6/<-0. 6) Genes called DE if at least 50% of representative probes are DE

R 2 = 0. 7437 R 2 = 0. 6569 P<0. 05 P<0. 001
Sources of inconsistent gene expression explained • GSU 2480 – Confirmed by q. RT-PCR to match a. RNA data • GSU 0964, 0966, 0967 – Operon of hypotheticals • GSU 0774, 0919 – Low sequence complexity • GSU 2959 – Genomice arrangement suggests the wrong strand was detected in the Amplicon array
Oligonucleotide design reduces cross hybridization due to regions of low sequence complexity • GSU 0774 – 70. 5% G+C content • GSU 0919 – 60. 4% G+C content – Multiple tetra- and tri- nucleotide repeats
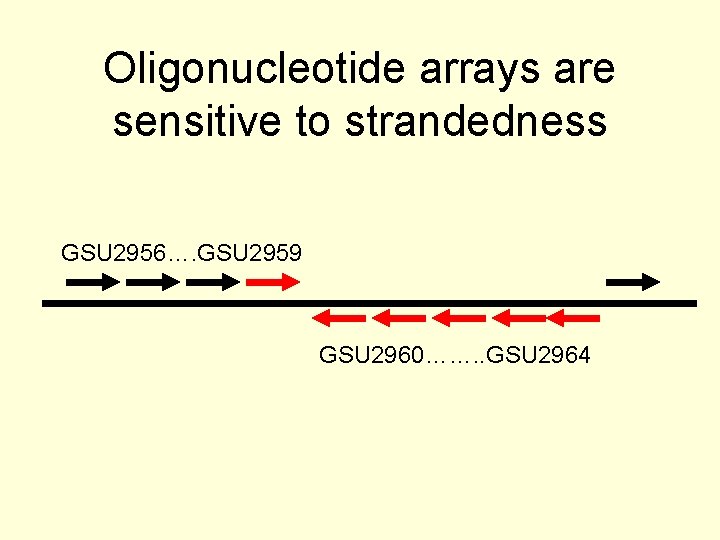
Oligonucleotide arrays are sensitive to strandedness GSU 2956…. GSU 2959 GSU 2960……. . GSU 2964

R 2 = 0. 8751
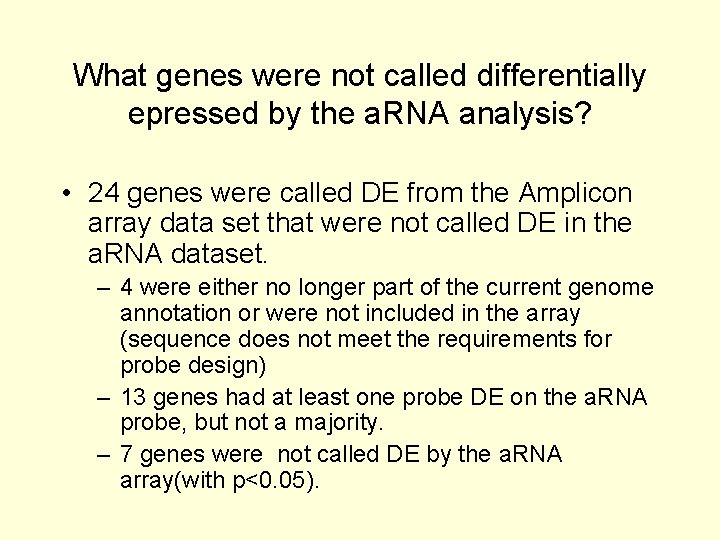
What genes were not called differentially epressed by the a. RNA analysis? • 24 genes were called DE from the Amplicon array data set that were not called DE in the a. RNA dataset. – 4 were either no longer part of the current genome annotation or were not included in the array (sequence does not meet the requirements for probe design) – 13 genes had at least one probe DE on the a. RNA probe, but not a majority. – 7 genes were not called DE by the a. RNA array(with p<0. 05).
Conclusions • Combimatrix Customarrays coupled with RNA amplification is a viable method for gene expression studies – Results correlate well with q. RT-PCR results and previously reported gene expression patterns under identical conditions • Combimatrix Oligonucleotide arrays provide several benefits – Differentiates between expression on +/- strand – Data suggests genes with regions of low sequence specificity are less prone to cross-hybridization due to the use of short oligonucleotides with unique hybridization characteristics – Low variance across technical and biological replicates increases the number of genes differentially expressed – Gasketed hybridization chamber design reduces handling time and improves variance across replicate slides.
$$ Combimatrix Customarray 12 k arrays are an affordable alternative to “Home brew” arrays • • • Increased specificity over pcr amplicon arrays No oligo design or purchase fee No oligo/amplicon validation or amplification No storage, tracking, or transferring prepared plates No printing – Pins, slides, plates, solutions, arrayer, labor – No validation, storage, post print processing • Slides may be stripped and used again (4 x) • Gasketed hybridization chamber design reduces handling time and improves variance across replicate slides. • Rigid design allows more automated spot finding

Possible Future Work • Develop electrasense technology in house? • Improve array design – Identify poorly responsive probes • Larger format arrays – More technical replicates, intergenic regions • Dual use arrays – Amplified or unamplified • Hyb and Seq technology – Resequence a region of the genome • Chip/Ch. IP analysis • Purchase a synthesizer?
- Slides: 21