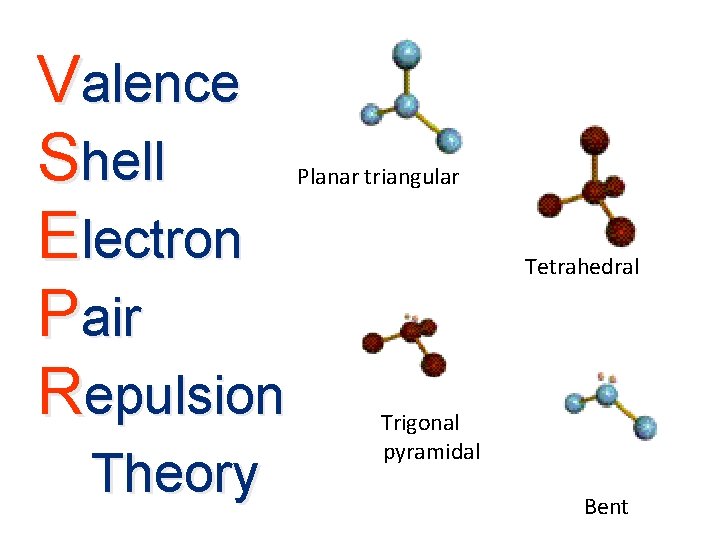
Valence Shell Electron Pair Repulsion Theory Planar triangular
- Slides: 20

Valence Shell Electron Pair Repulsion Theory Planar triangular Tetrahedral Trigonal pyramidal Bent

What is the VSEPR Theory? A model for describing the shapes of molecules whose main postulate is that the structure around a given atom is determined by minimizing the electron pair repulsion • The VSEPR Theory is used to predict the shapes of molecules based on the repulsion of the bonding and non-bonding electrons in the molecule. • The shape is determined by the number of bonding and non-bonding electrons in the molecule.

Repulsion strengths • Lone pair -Lone pair Bond pair Bond pair-Bond pair Triple bond >double bond >single bond

Steps in the prediction of geometry • Determine the central atom. • Draw the electron dot structure and bar diagram • Find arrangement of electron pairs. • Find arrangement of bonding pairs. • Determine the geometry based on of bonding pairs.

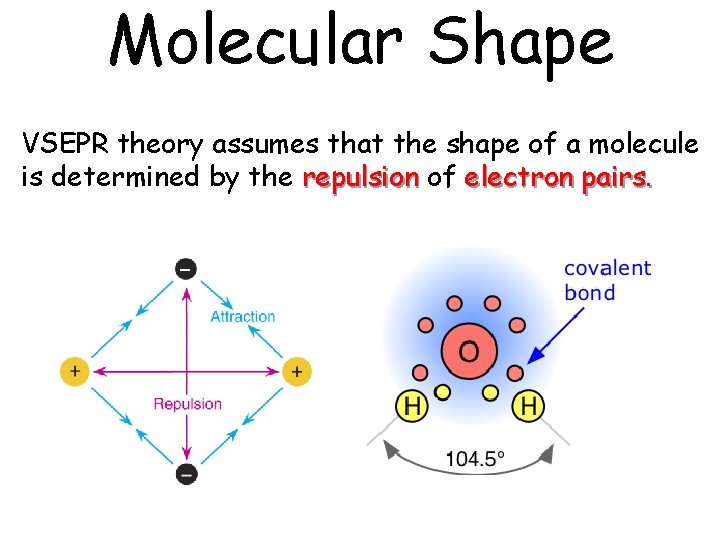
Molecular Shape VSEPR theory assumes that the shape of a molecule is determined by the repulsion of electron pairs.

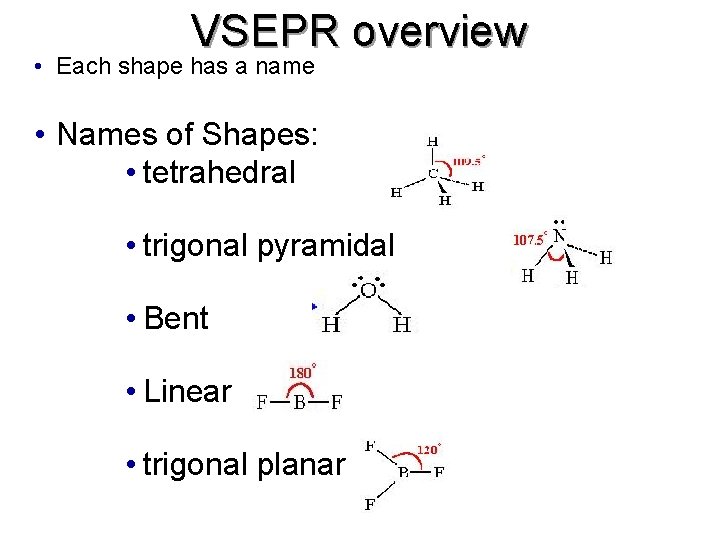
VSEPR overview • Each shape has a name • Names of Shapes: • tetrahedral • trigonal pyramidal • Bent • Linear • trigonal planar

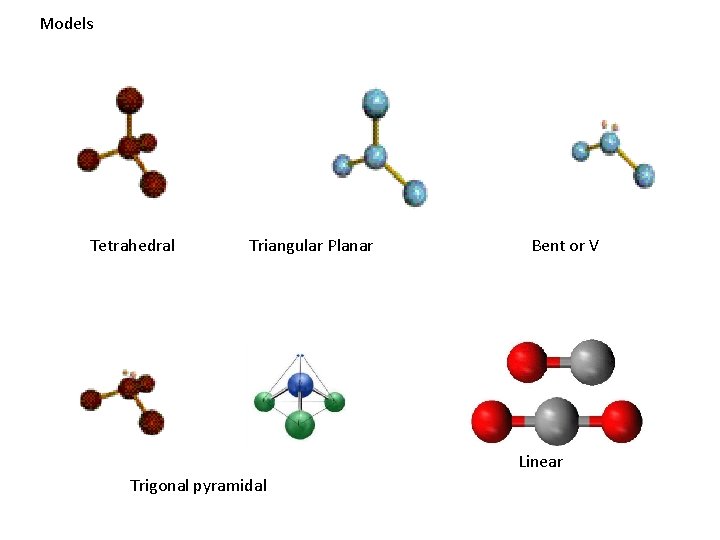
Models Tetrahedral Triangular Planar Bent or V Linear Trigonal pyramidal

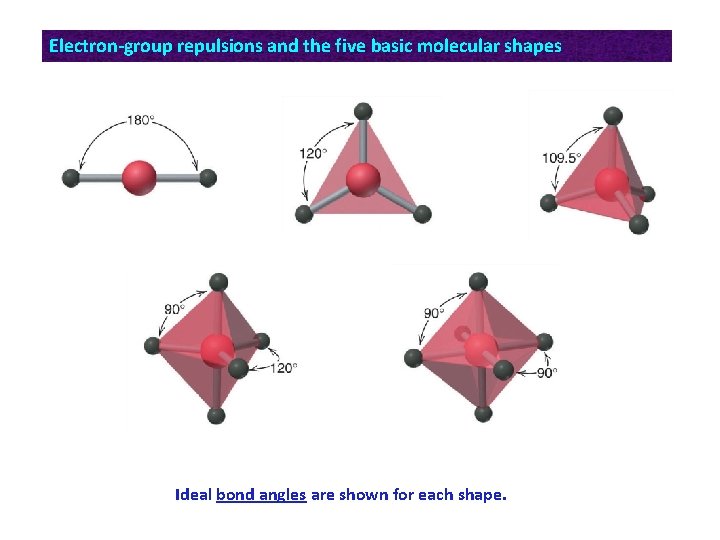
Electron-group repulsions and the five basic molecular shapes Ideal bond angles are shown for each shape.

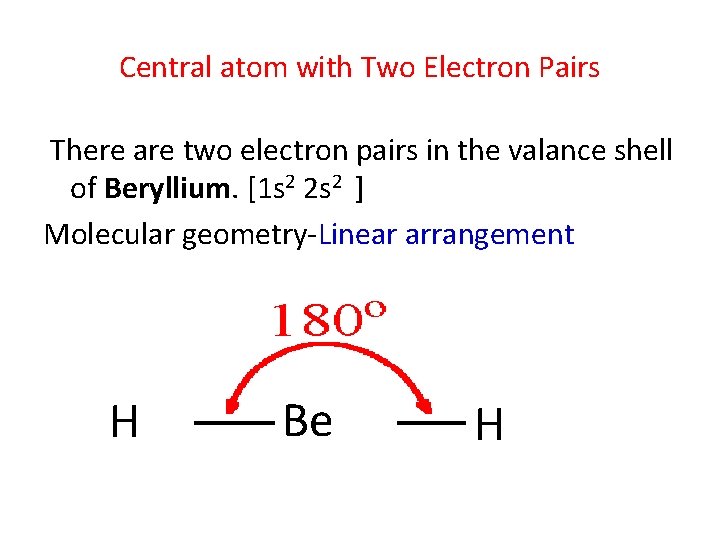
Central atom with Two Electron Pairs There are two electron pairs in the valance shell of Beryllium. [1 s 2 2 s 2 ] Molecular geometry-Linear arrangement H Be H

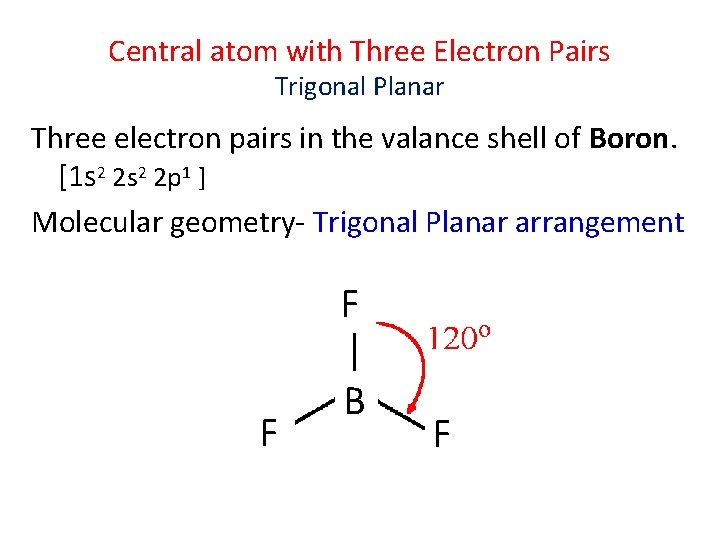
Central atom with Three Electron Pairs Trigonal Planar Three electron pairs in the valance shell of Boron. [1 s 2 2 p 1 ] Molecular geometry- Trigonal Planar arrangement F F B F

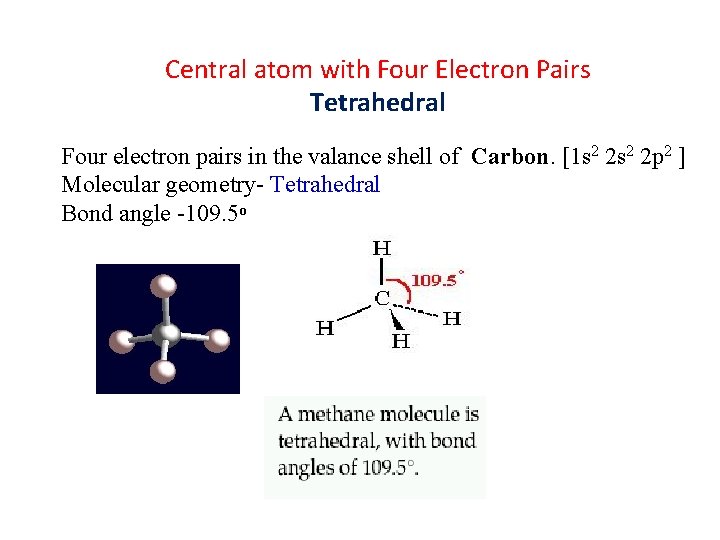
Central atom with Four Electron Pairs Tetrahedral Four electron pairs in the valance shell of Carbon. [1 s 2 2 p 2 ] Molecular geometry- Tetrahedral Bond angle -109. 5⁰

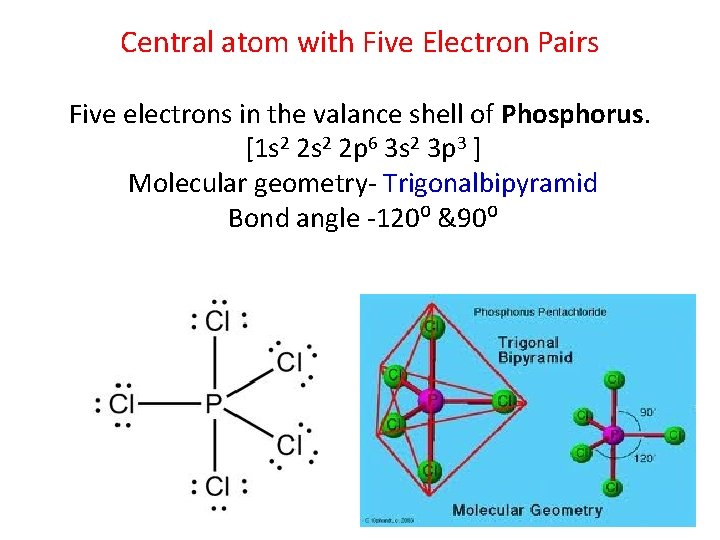
Central atom with Five Electron Pairs Five electrons in the valance shell of Phosphorus. [1 s 2 2 p 6 3 s 2 3 p 3 ] Molecular geometry- Trigonalbipyramid Bond angle -120⁰ &90⁰

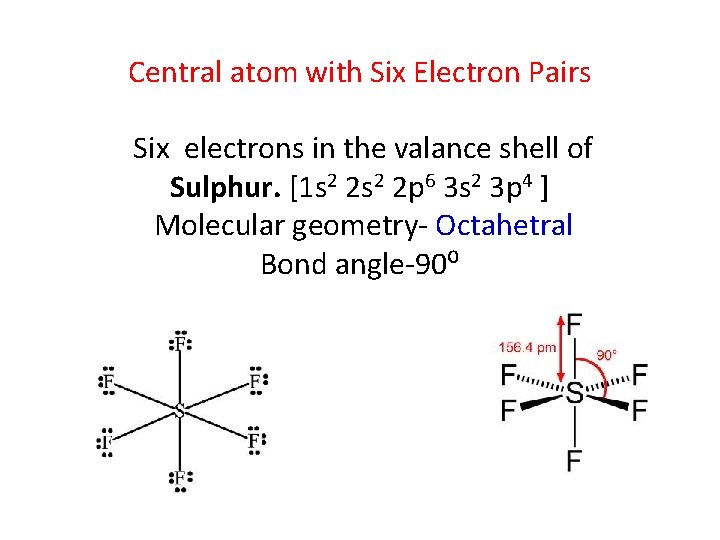
Central atom with Six Electron Pairs Six electrons in the valance shell of Sulphur. [1 s 2 2 p 6 3 s 2 3 p 4 ] Molecular geometry- Octahetral Bond angle-90⁰

SHAPES OF MOLECULES CONTAINING BONDED PAIRS AS WELL AS LONE PAIRS OF ELECTRONS

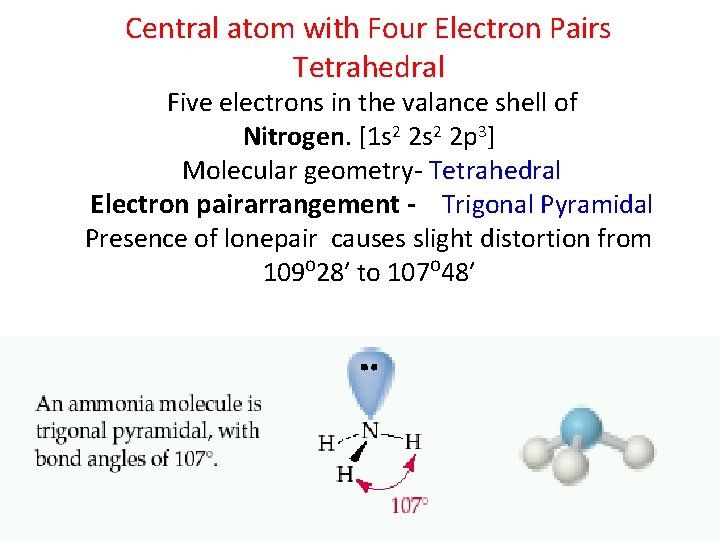
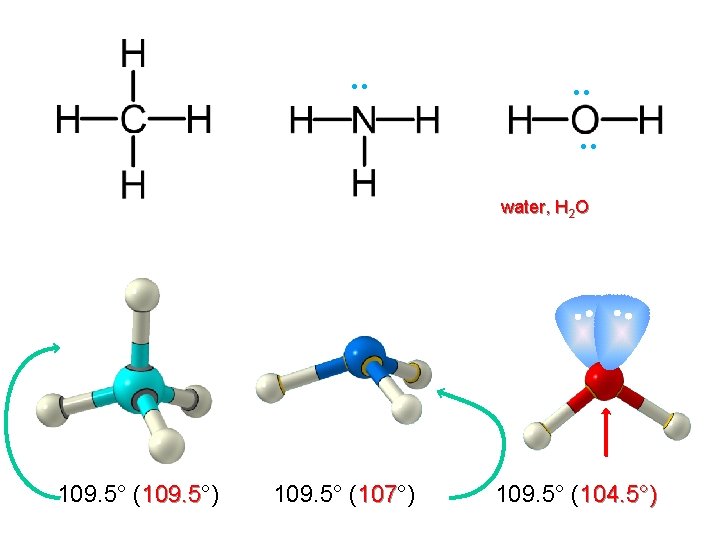
Central atom with Four Electron Pairs Tetrahedral Five electrons in the valance shell of Nitrogen. [1 s 2 2 p 3] Molecular geometry- Tetrahedral Electron pairarrangement - Trigonal Pyramidal Presence of lonepair causes slight distortion from 109⁰ 28′ to 107⁰ 48′

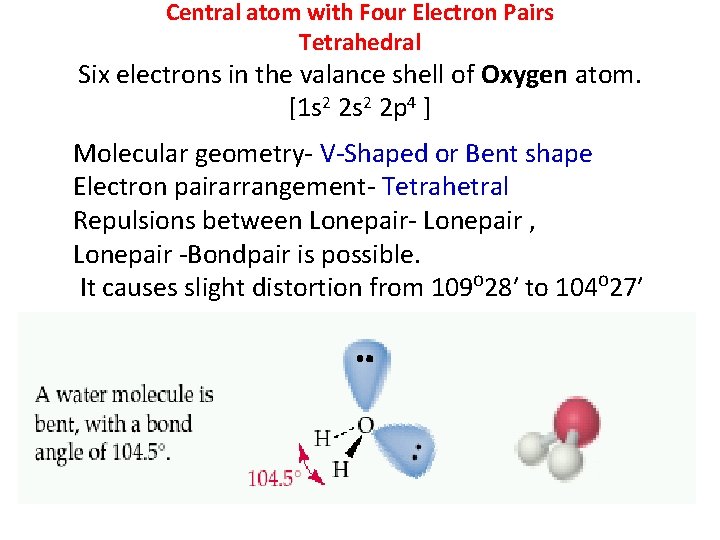
Central atom with Four Electron Pairs Tetrahedral Six electrons in the valance shell of Oxygen atom. [1 s 2 2 p 4 ] Molecular geometry- V-Shaped or Bent shape Electron pairarrangement- Tetrahetral Repulsions between Lonepair- Lonepair , Lonepair -Bondpair is possible. It causes slight distortion from 109⁰ 28′ to 104⁰ 27′

. . . water, H 2 O 109. 5° (109. 5°) 109. 5° (107°) 109. 5° (104. 5°)

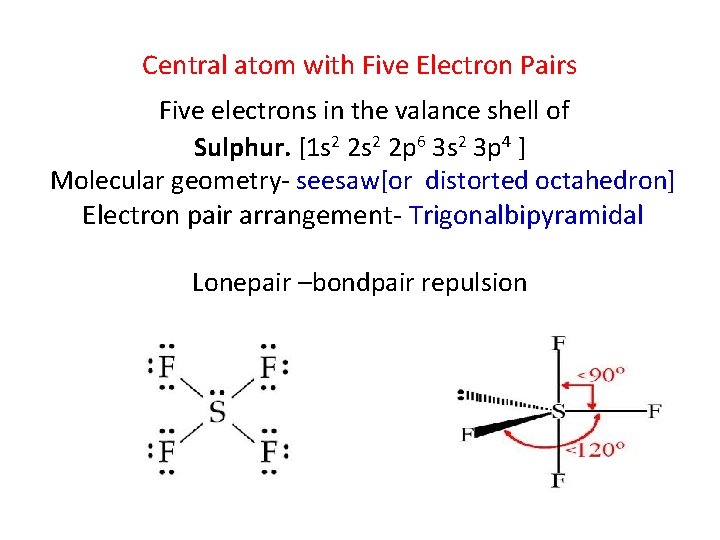
Central atom with Five Electron Pairs Five electrons in the valance shell of Sulphur. [1 s 2 2 p 6 3 s 2 3 p 4 ] Molecular geometry- seesaw[or distorted octahedron] Electron pair arrangement- Trigonalbipyramidal Lonepair –bondpair repulsion

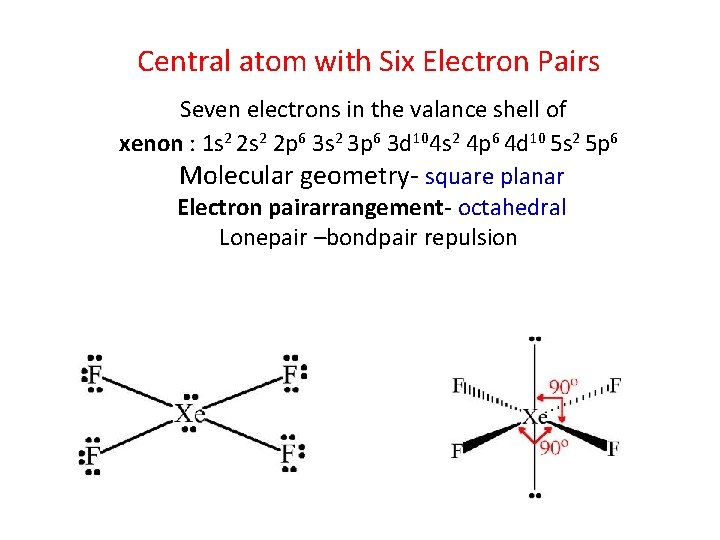
Central atom with Six Electron Pairs Seven electrons in the valance shell of xenon : 1 s 2 2 p 6 3 s 2 3 p 6 3 d 104 s 2 4 p 6 4 d 10 5 s 2 5 p 6 Molecular geometry- square planar Electron pairarrangement- octahedral Lonepair –bondpair repulsion

Limitations of VSEPR • It fails to predict the shapes of isoelectronic species[CH 4&NH 4+] and transition metal compounds. • This model does not take relative sizes of substituents. • Unable to explain atomic orbitals overlap.