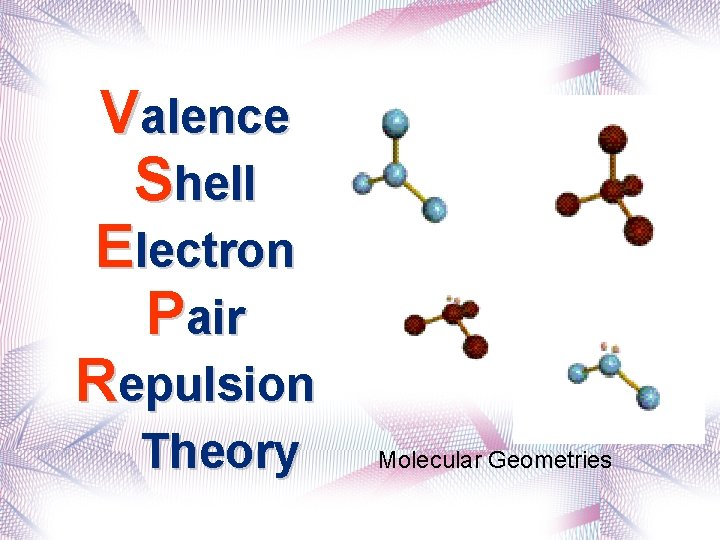
Valence Shell Electron Pair Repulsion Theory Molecular Geometries




- Slides: 37

Valence Shell Electron Pair Repulsion Theory Molecular Geometries

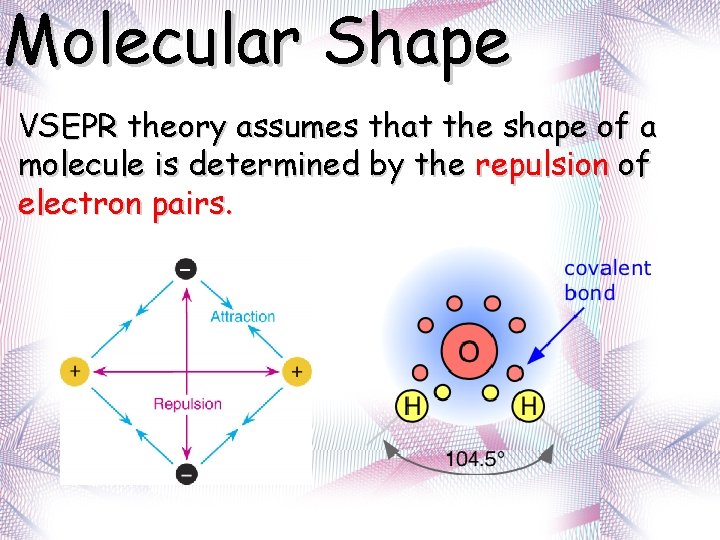
Molecular Shape VSEPR theory assumes that the shape of a molecule is determined by the repulsion of electron pairs.

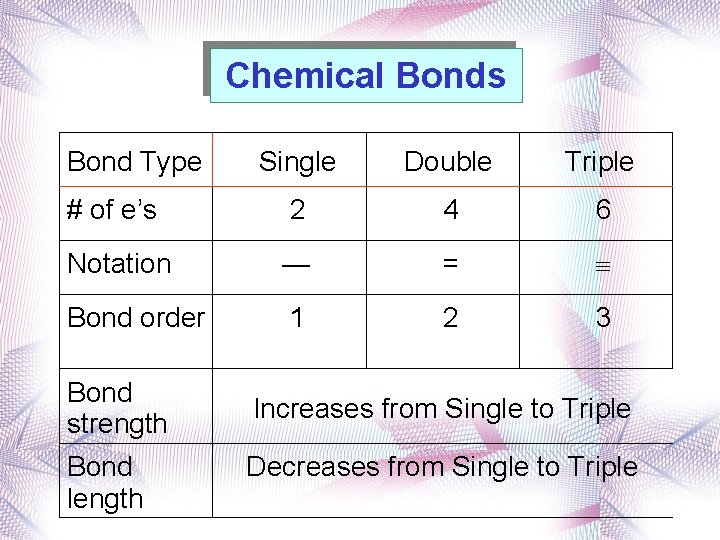
Chemical Bonds Bond Type Single Double Triple # of e’s 2 4 6 Notation — = Bond order 1 2 3 Bond strength Bond length Increases from Single to Triple Decreases from Single to Triple

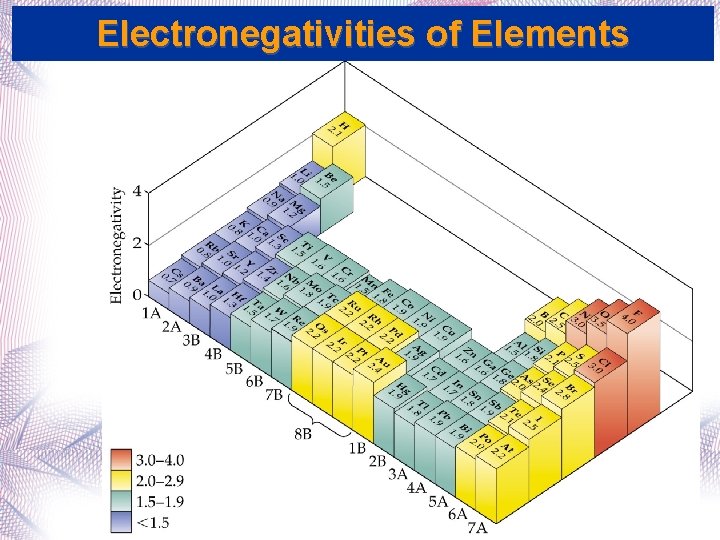
Bond Polarity and Electronegativity • Electronegativity: The ability of one atom in a molecule to attract other electrons. • Electronegativities range from 0. 7 (Cs) to 4. 0 (F). • Trend in Electronegativity increases… • From left to right across a period • From bottom to top in a group.

Electronegativities of Elements

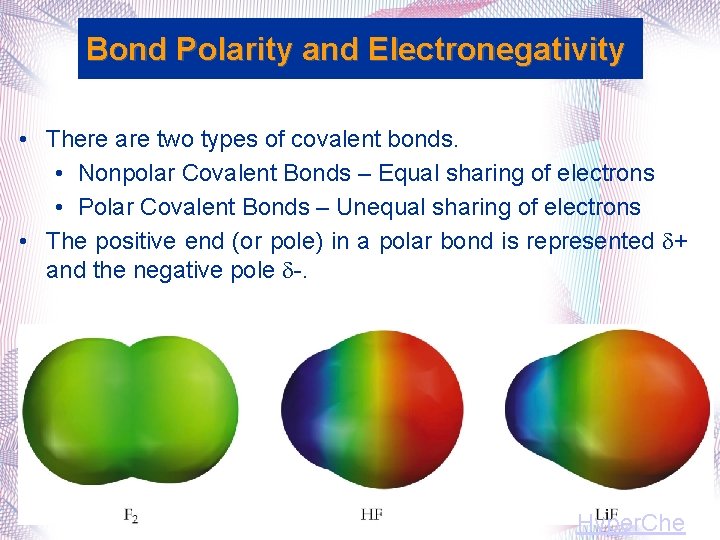
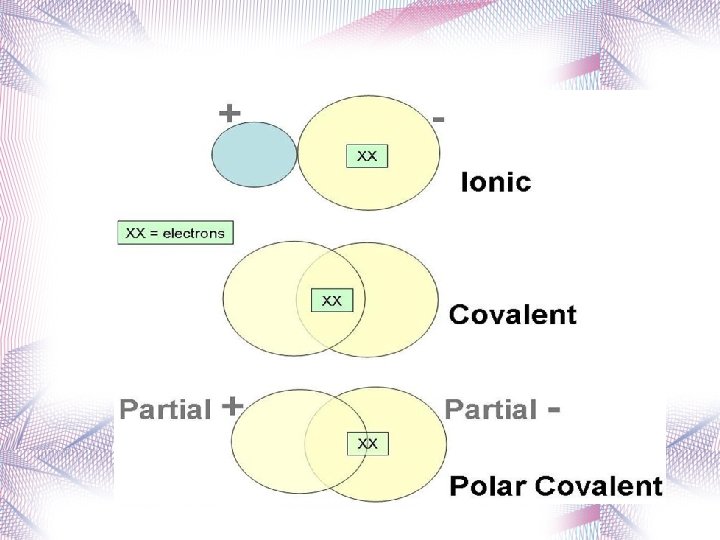
Bond Polarity and Electronegativity • There are two types of covalent bonds. • Nonpolar Covalent Bonds – Equal sharing of electrons • Polar Covalent Bonds – Unequal sharing of electrons • The positive end (or pole) in a polar bond is represented + and the negative pole . Hyper. Che


Polar Covalent Bond • A Polar Covalent Bond is unequal sharing of electrons between two atoms (H-Cl) • In a polar covalent bond, one atom typically has a negative charge, and the other atom has a positive charge

Nonpolar Covalent Bond • A Nonpolar Covalent Bond is an equal sharing of electrons between two atoms (Cl Cl, N N, O O)


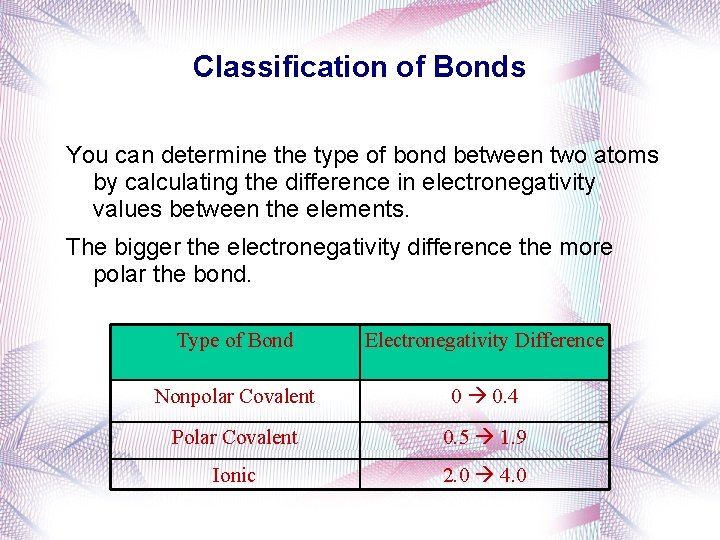
Classification of Bonds You can determine the type of bond between two atoms by calculating the difference in electronegativity values between the elements. The bigger the electronegativity difference the more polar the bond. Type of Bond Electronegativity Difference Nonpolar Covalent 0 0. 4 Polar Covalent 0. 5 1. 9 Ionic 2. 0 4. 0

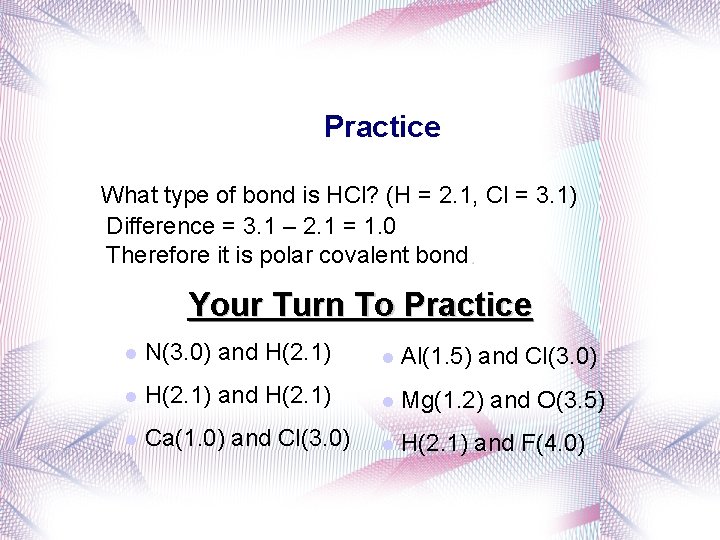
Practice What type of bond is HCl? (H = 2. 1, Cl = 3. 1) Difference = 3. 1 – 2. 1 = 1. 0 Therefore it is polar covalent bond. Your Turn To Practice l N(3. 0) and H(2. 1) l Al(1. 5) and Cl(3. 0) l H(2. 1) and H(2. 1) l Mg(1. 2) and O(3. 5) l Ca(1. 0) and Cl(3. 0) l H(2. 1) and F(4. 0)

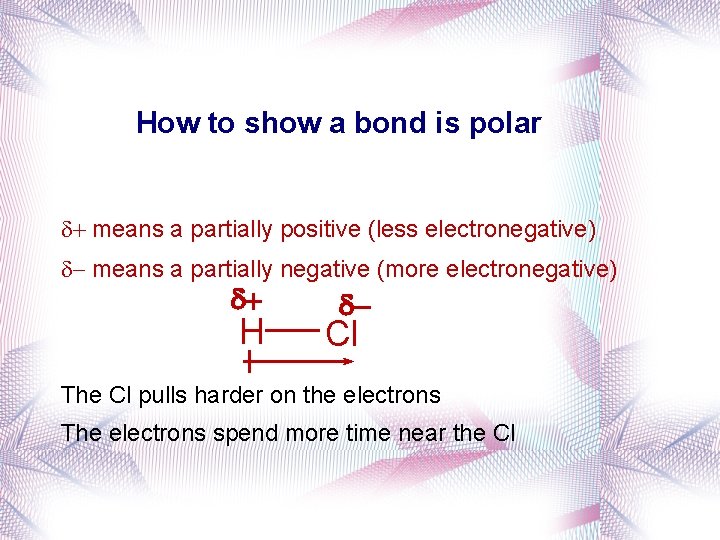
How to show a bond is polar + means a partially positive (less electronegative) - means a partially negative (more electronegative) d+ H d- Cl The Cl pulls harder on the electrons The electrons spend more time near the Cl

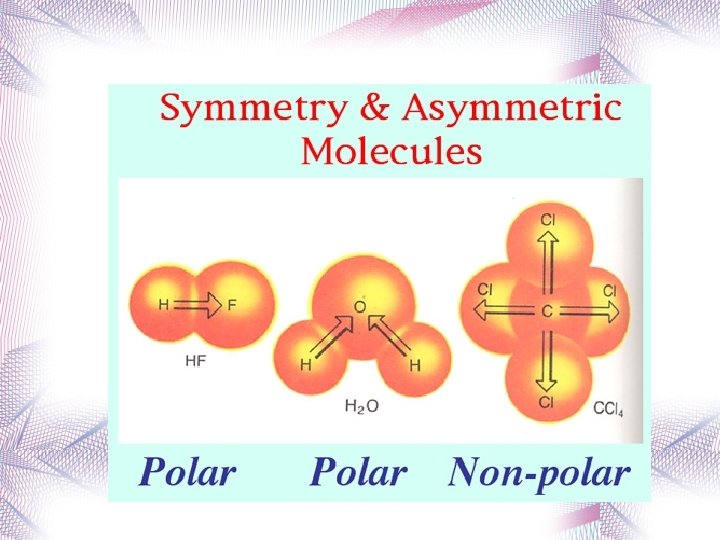
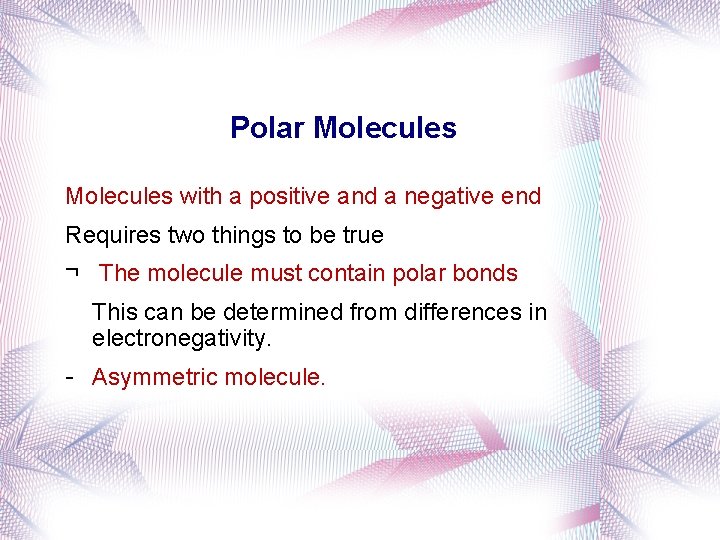
Polar Molecules with a positive and a negative end Requires two things to be true ¬ The molecule must contain polar bonds This can be determined from differences in electronegativity. Asymmetric molecule.

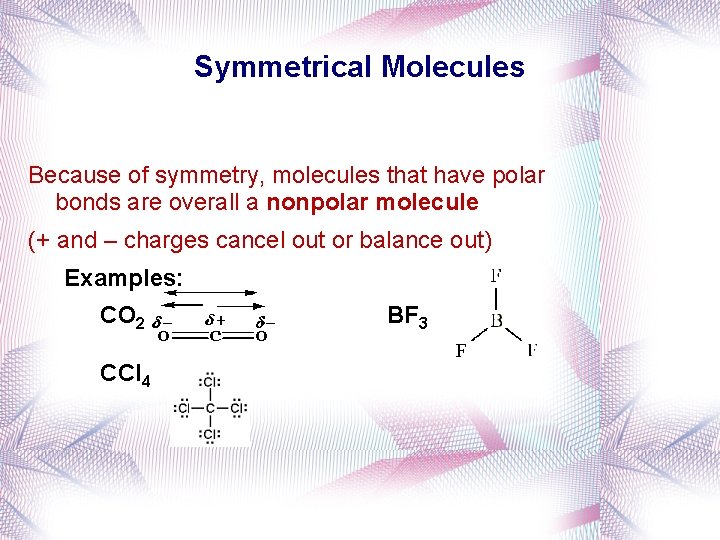
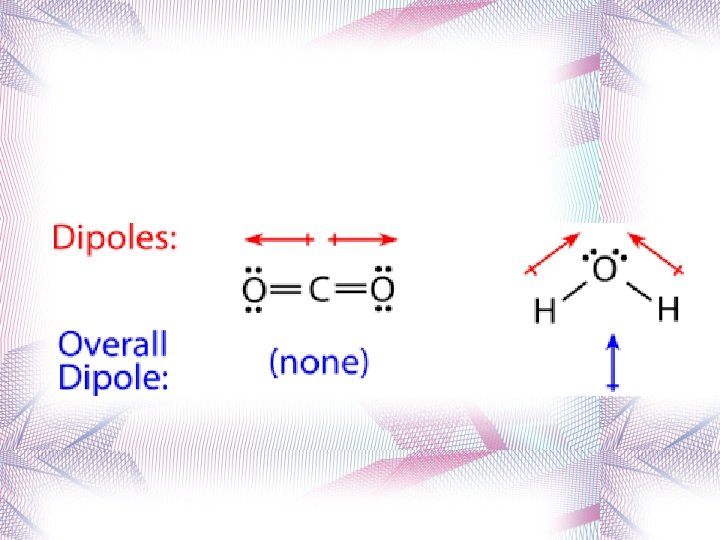
Symmetrical Molecules Because of symmetry, molecules that have polar bonds are overall a nonpolar molecule (+ and – charges cancel out or balance out) Examples: CO 2 CCl 4 BF 3

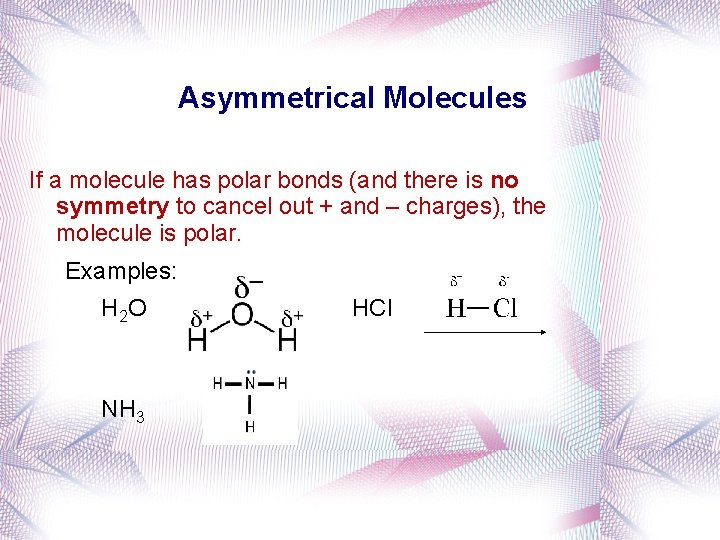
Asymmetrical Molecules If a molecule has polar bonds (and there is no symmetry to cancel out + and – charges), the molecule is polar. Examples: H 2 O NH 3 HCl

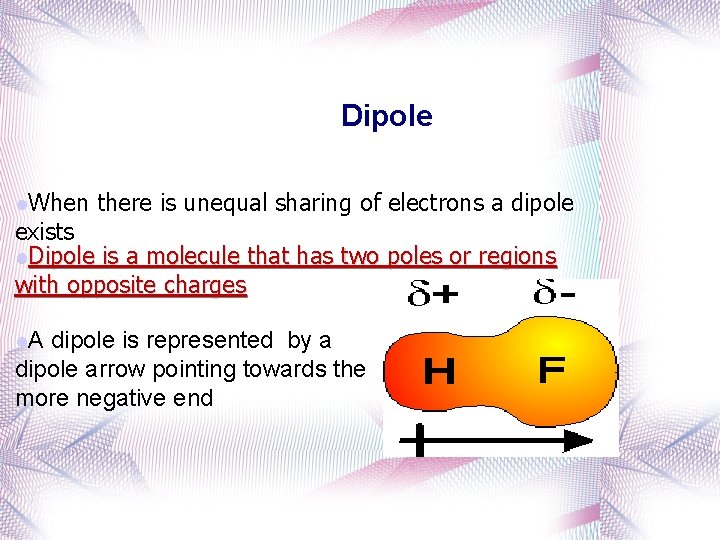
Dipole l. When there is unequal sharing of electrons a dipole exists l. Dipole is a molecule that has two poles or regions with opposite charges l. A dipole is represented by a dipole arrow pointing towards the more negative end


Is it Polar? HF H 2 O NH 3 CF 4 CO 2

Exceptions to the Octet Rule Central Atoms Having Less than an Octet • Relatively rare. • Molecules with less than an octet are typical for compounds of Groups 1 A, 2 A, and 3 A. • Most typical example is BF 3.

Exceptions to the Octet Rule Central Atoms Having More than an Octet • This is the largest class of exceptions. • Atoms from the 3 rd period onwards can accommodate more than an octet. • Beyond the third period, the d orbitals are low enough in energy to participate in bonding and accept the extra electron density. Hyper. Chem

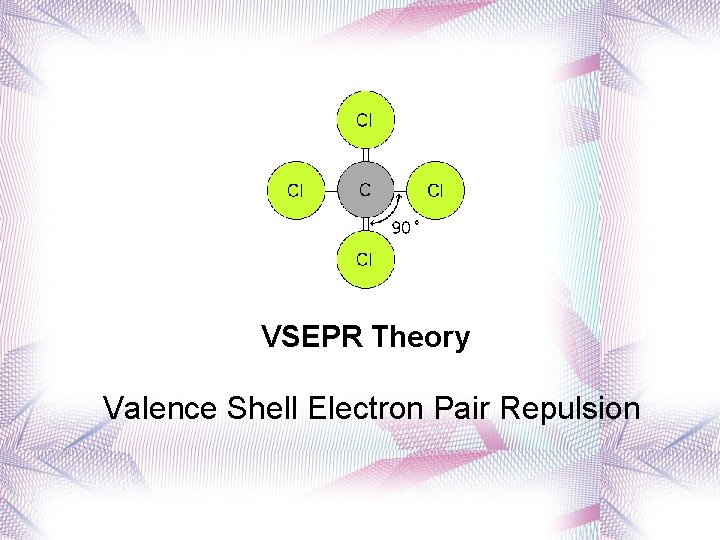
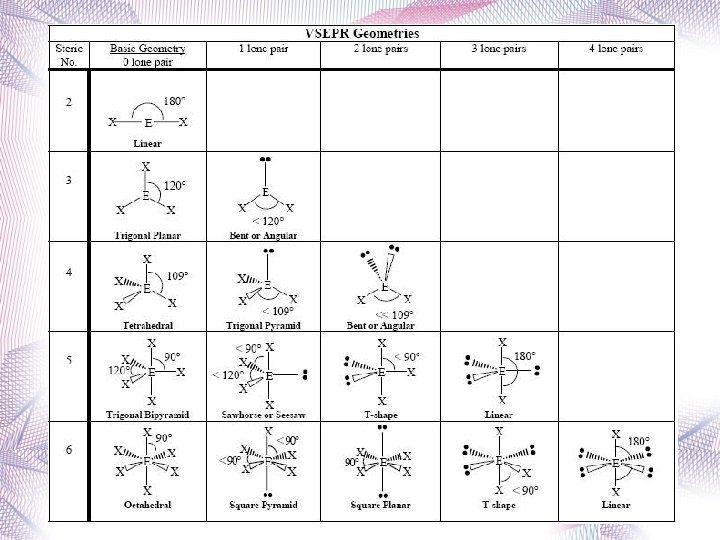
VSEPR Theory Valence Shell Electron Pair Repulsion

VSEPR Theory • Predicts the molecular shape of a bonded molecule. • Electron pairs in the valence shell of an atom tend to orient themselves so that the total energy is minimized. • Electrons around the central atom arrange themselves as far apart from each other as possible. • Unshared pairs of electrons (lone pairs) on the central atom repel the most.

VSEPR Theory Because lone pairs of electrons are spread out more broadly than bond pairs, repulsions are greatest between two lone pairs, intermediate between a lone pair and a bond pair, and weakest between two bonding pairs of electrons. Repulsive forces decrease rapidly with increasing angle between the central atom and the outside atoms. Greatest at 90 o, much weaker at 120 o, and very weak at 180 o.

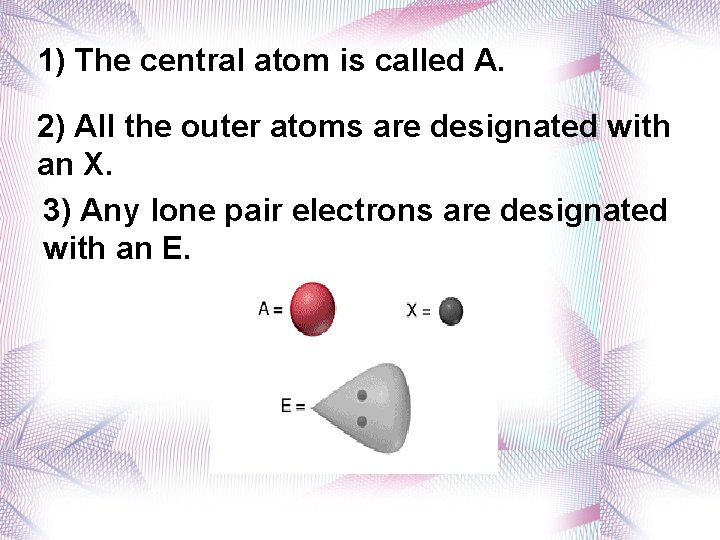
1) The central atom is called A. 2) All the outer atoms are designated with an X. 3) Any lone pair electrons are designated with an E.

Compounds with paired electrons around the central atom.

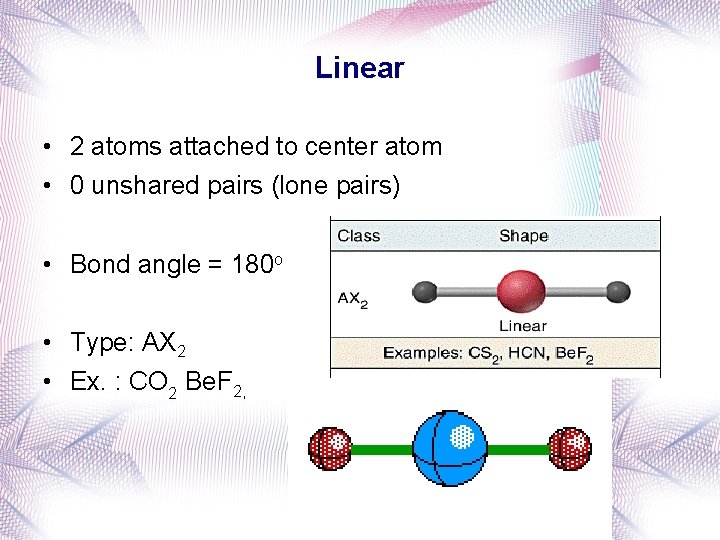
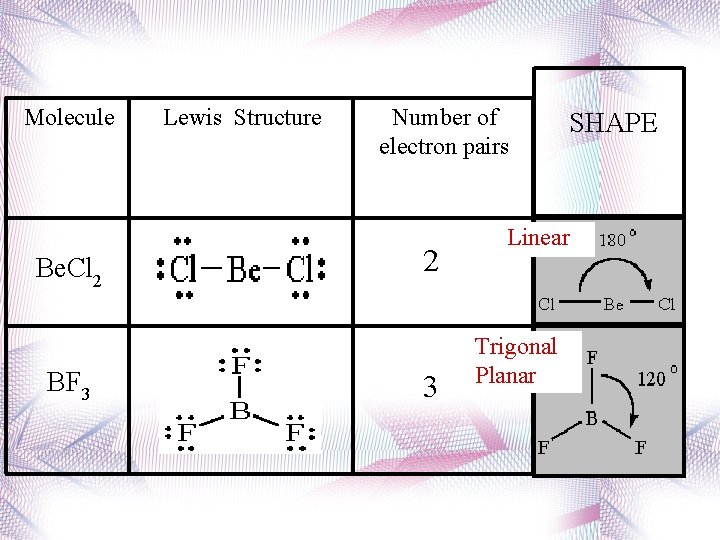
Linear • 2 atoms attached to center atom • 0 unshared pairs (lone pairs) • Bond angle = 180 o • Type: AX 2 • Ex. : CO 2 Be. F 2,

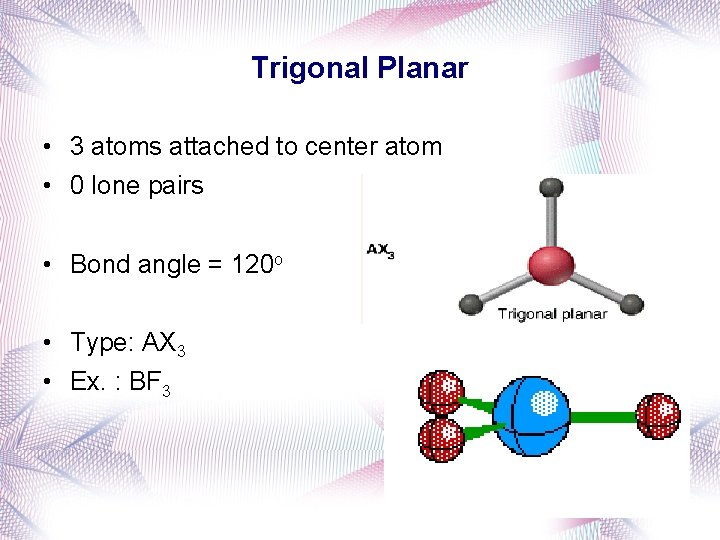
Trigonal Planar • 3 atoms attached to center atom • 0 lone pairs • Bond angle = 120 o • Type: AX 3 • Ex. : BF 3

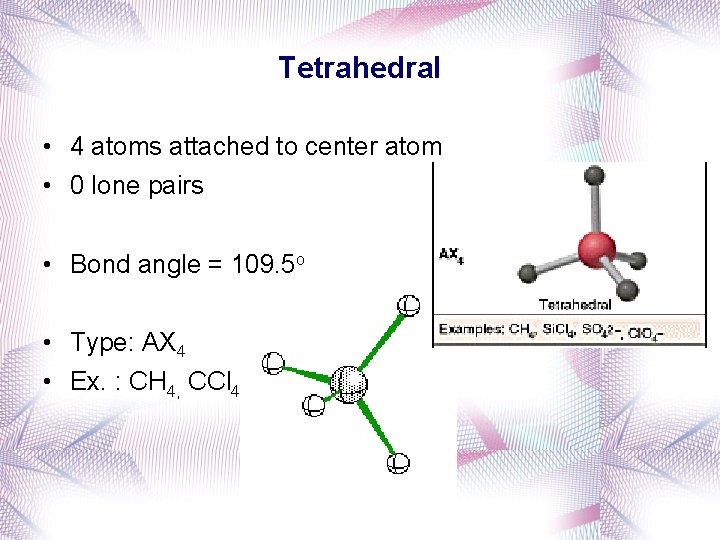
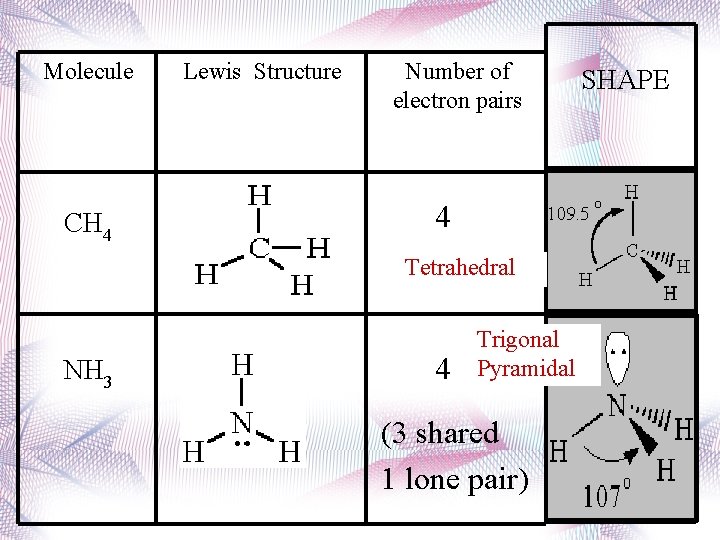
Tetrahedral • 4 atoms attached to center atom • 0 lone pairs • Bond angle = 109. 5 o • Type: AX 4 • Ex. : CH 4, CCl 4

Compounds with unshared (lone) pairs of electrons around the central atom.

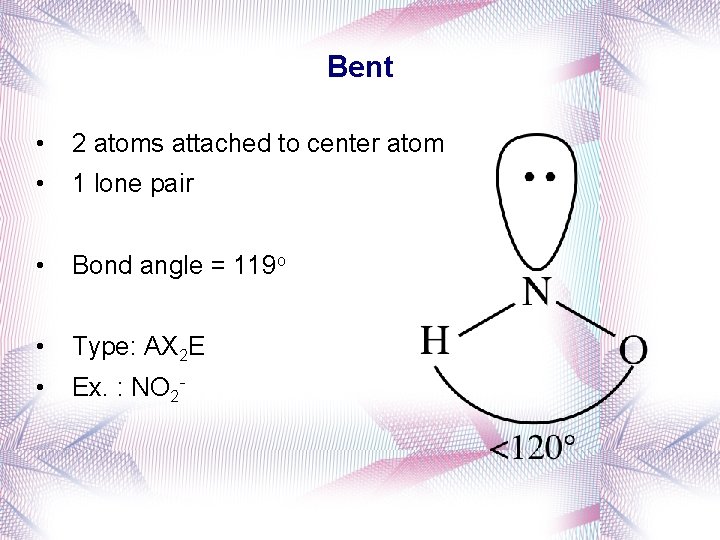
Bent • 2 atoms attached to center atom • 1 lone pair • Bond angle = 119 o • Type: AX 2 E • Ex. : NO 2

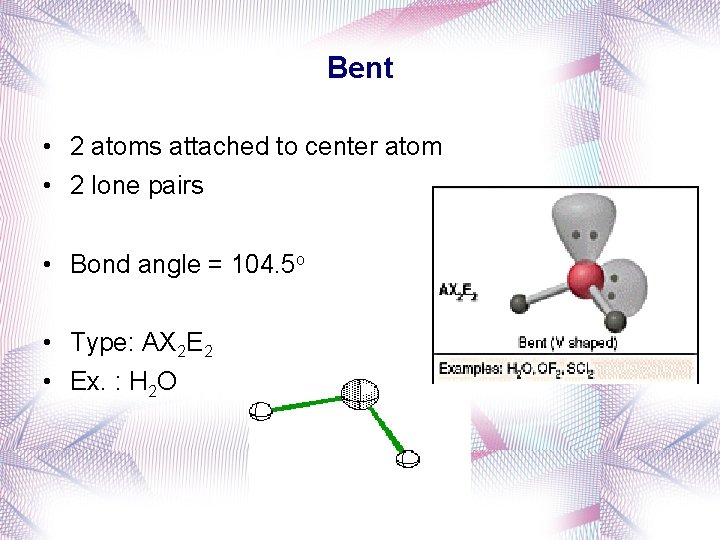
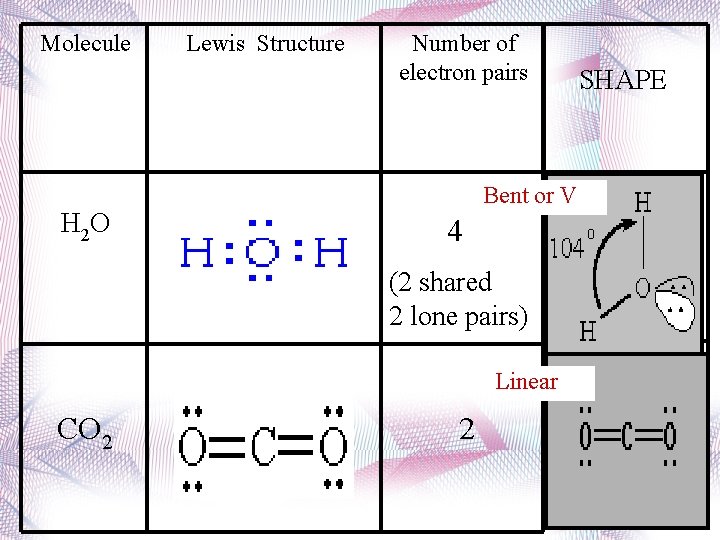
Bent • 2 atoms attached to center atom • 2 lone pairs • Bond angle = 104. 5 o • Type: AX 2 E 2 • Ex. : H 2 O

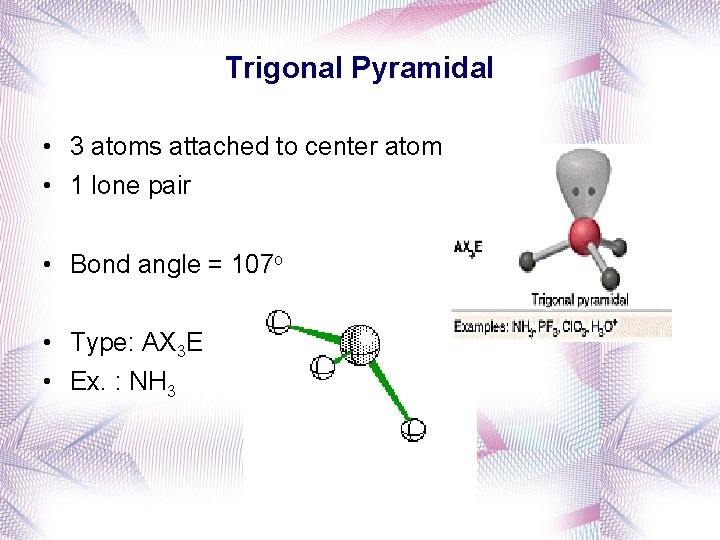
Trigonal Pyramidal • 3 atoms attached to center atom • 1 lone pair • Bond angle = 107 o • Type: AX 3 E • Ex. : NH 3


Molecule CH 4 Lewis Structure Number of electron pairs 4 Tetrahedral NH 3 4 Trigonal Pyramidal (3 shared 1 lone pair) SHAPE

Molecule H 2 O Lewis Structure Number of electron pairs Bent or V 4 (2 shared 2 lone pairs) Linear CO 2 2 SHAPE

Molecule Be. Cl 2 BF 3 Lewis Structure Number of electron pairs 2 3 SHAPE Linear Trigonal Planar