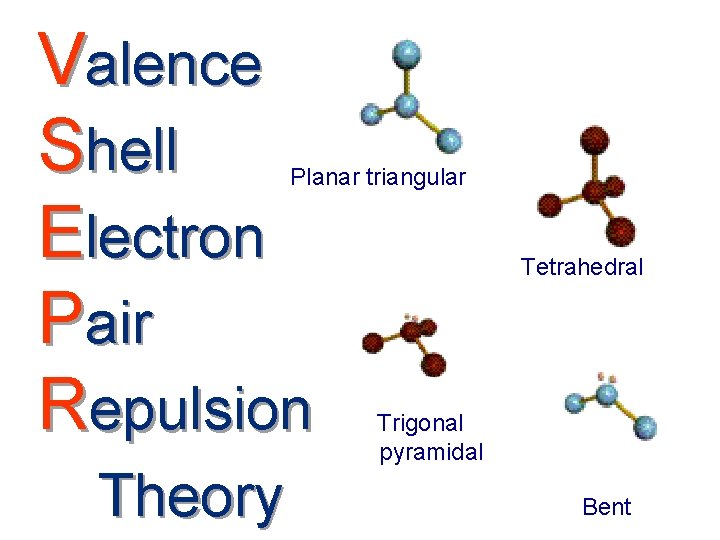
Valence Shell Electron Pair Repulsion Planar triangular Theory








- Slides: 29

Valence Shell Electron Pair Repulsion Planar triangular Theory Tetrahedral Trigonal pyramidal Bent

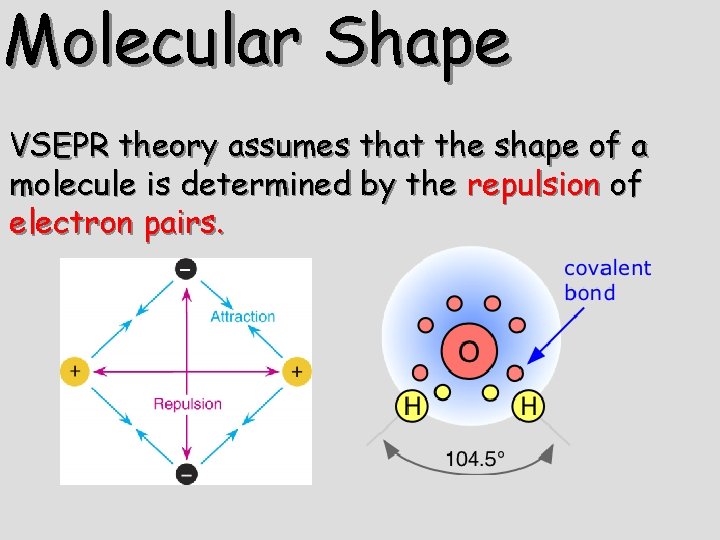
Molecular Shape VSEPR theory assumes that the shape of a molecule is determined by the repulsion of electron pairs.

VSEPR Theory • Based on Electron Dot (Lewis structures) • Theory predicts shapes of compounds • abbreviated VSEPR • VSEPR (pronounced “vesper”) stands for Valence Shell Electron Pair Repulsion • VSEPR predicts shapes based on electron pairs repelling (in bonds or by themselves) • Electrons around central nucleus each other. So, structures maximally spread out repel have atoms

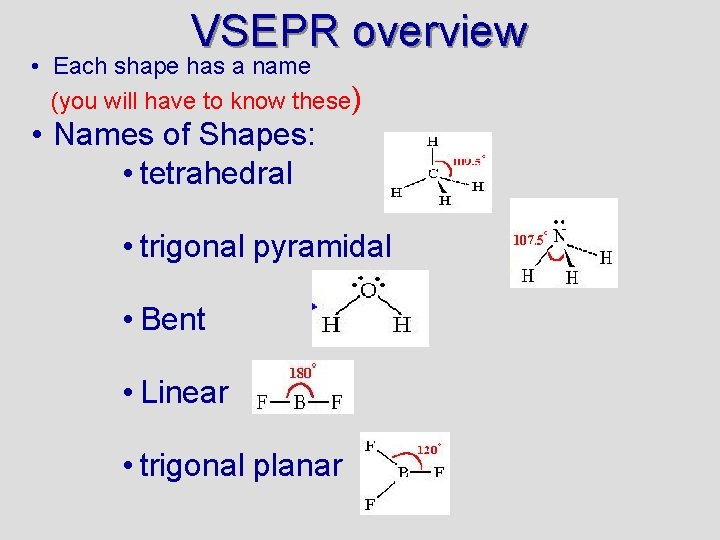
VSEPR overview • Each shape has a name (you will have to know these) • Names of Shapes: • tetrahedral • trigonal pyramidal • Bent • Linear • trigonal planar

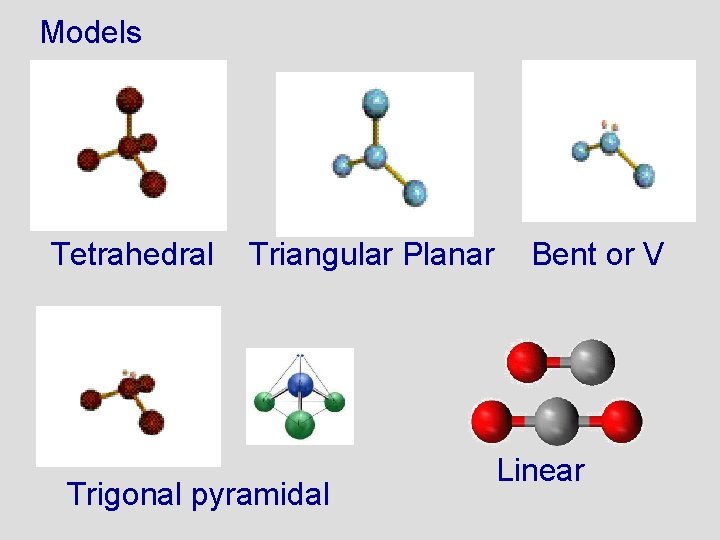
Models Tetrahedral Triangular Planar Trigonal pyramidal Bent or V Linear

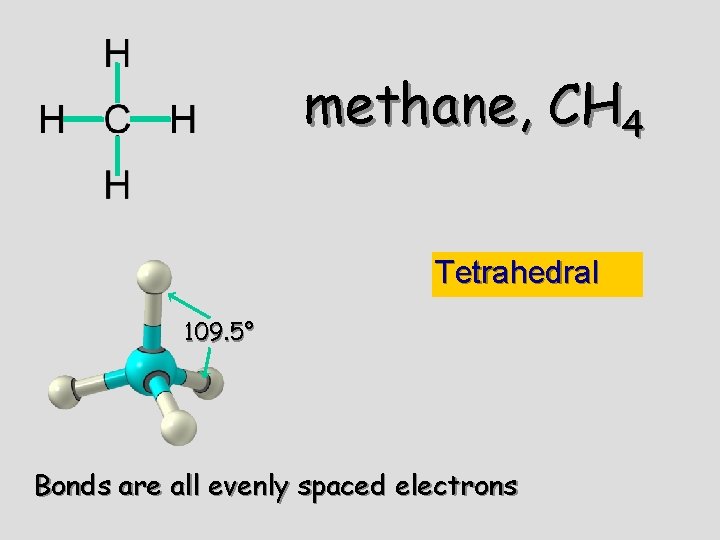
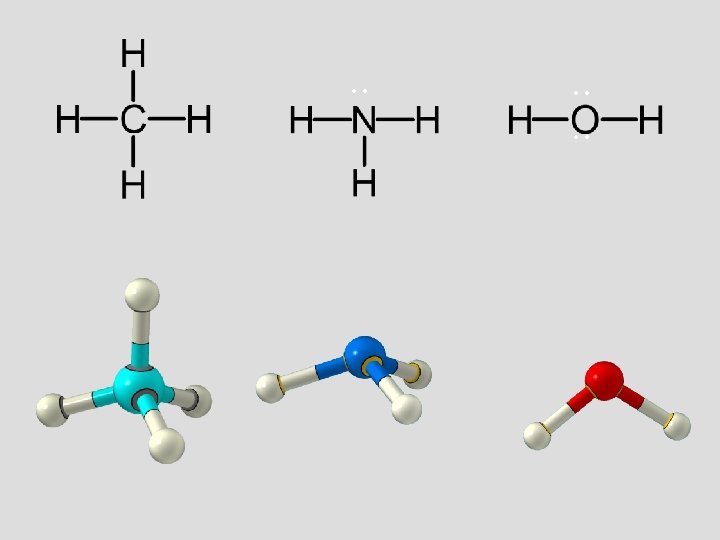
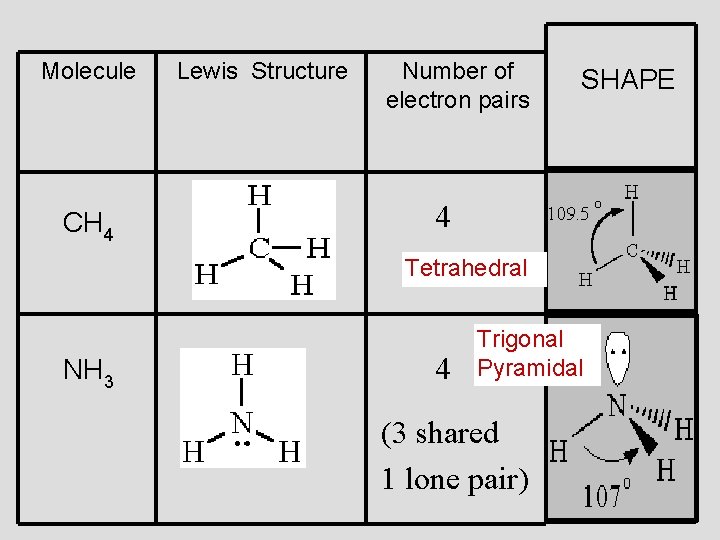
methane, CH 4 Tetrahedral 109. 5° Bonds are all evenly spaced electrons

. . . . ammonia NH 3 Trigonal Pyramidal Less repulsion between the bonding pairs of electrons

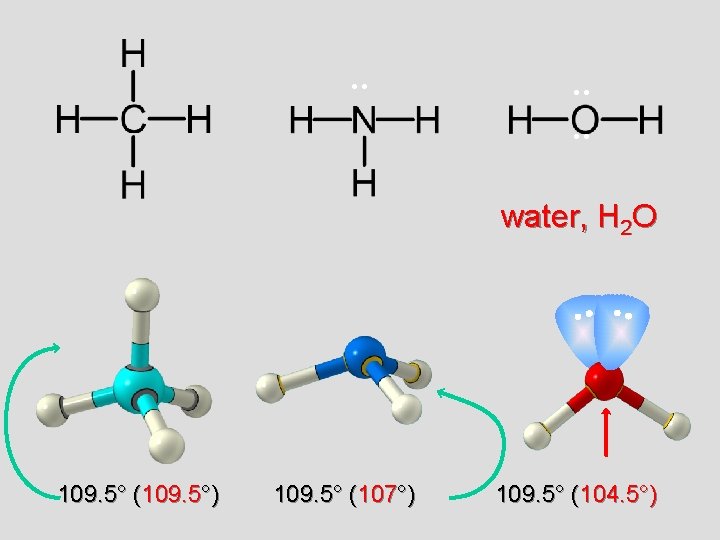
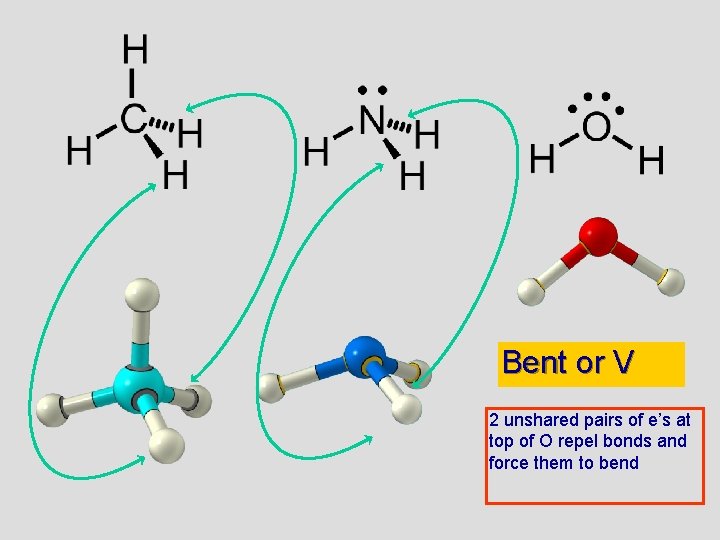
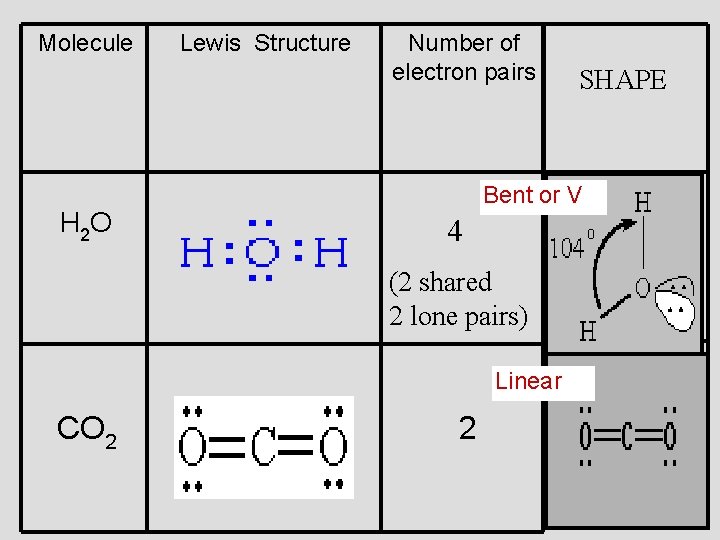
. . . water, H 2 O 109. 5° (109. 5°) 109. 5° (107°) 109. 5° (104. 5°)


Bent or V 2 unshared pairs of e’s at top of O repel bonds and force them to bend

Molecule CH 4 Lewis Structure Number of electron pairs SHAPE 4 Tetrahedral NH 3 4 Trigonal Pyramidal (3 shared 1 lone pair)

Molecule H 2 O Lewis Structure Number of electron pairs Bent or V 4 (2 shared 2 lone pairs) Linear CO 2 SHAPE 2

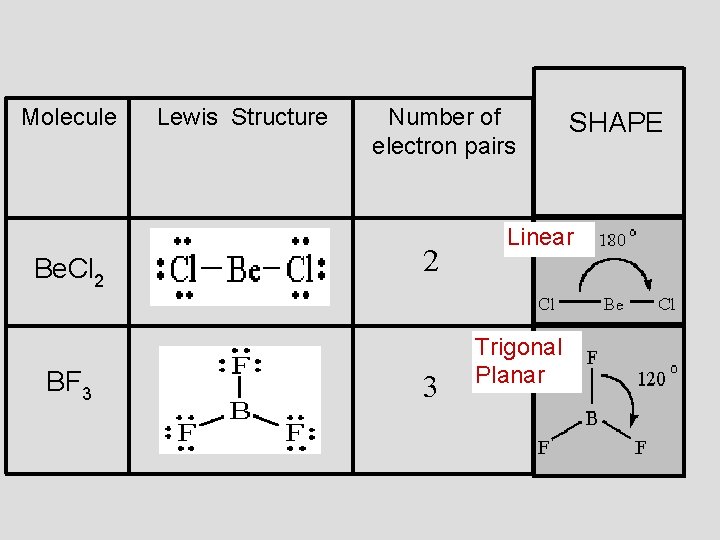
Molecule Be. Cl 2 BF 3 Lewis Structure Number of electron pairs 2 3 SHAPE Linear Trigonal Planar

HYBRIDIZATION

Hybridization – mixing of two or more atomic orbitals to form a new set of hybrid orbitals. 1. Mix at least 2 nonequivalent atomic orbitals (e. g. s and p). Hybrid orbitals have very different shape from original atomic orbitals. 2. Number of hybrid orbitals is equal to number of pure atomic orbitals used in the hybridization process. 3. Covalent bonds are formed by: a. Overlap of hybrid orbitals with atomic orbitals b. Overlap of hybrid orbitals with other hybrid orbitals 10. 4

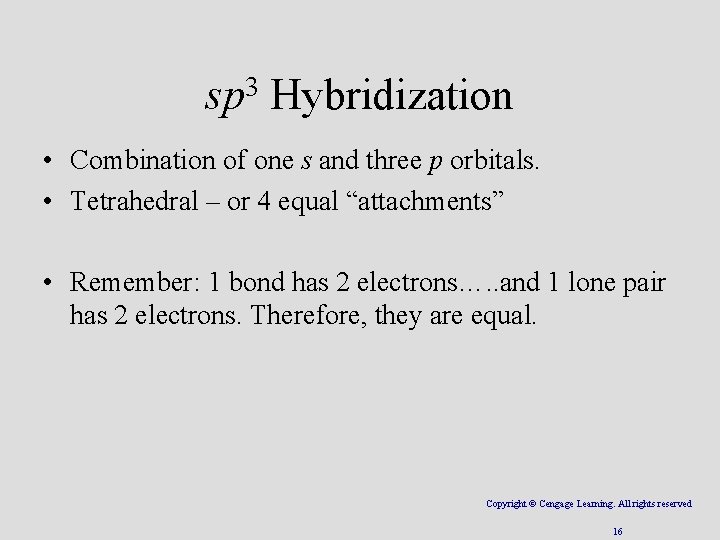
3 sp Hybridization • Combination of one s and three p orbitals. • Tetrahedral – or 4 equal “attachments” • Remember: 1 bond has 2 electrons…. . and 1 lone pair has 2 electrons. Therefore, they are equal. Copyright © Cengage Learning. All rights reserved 16

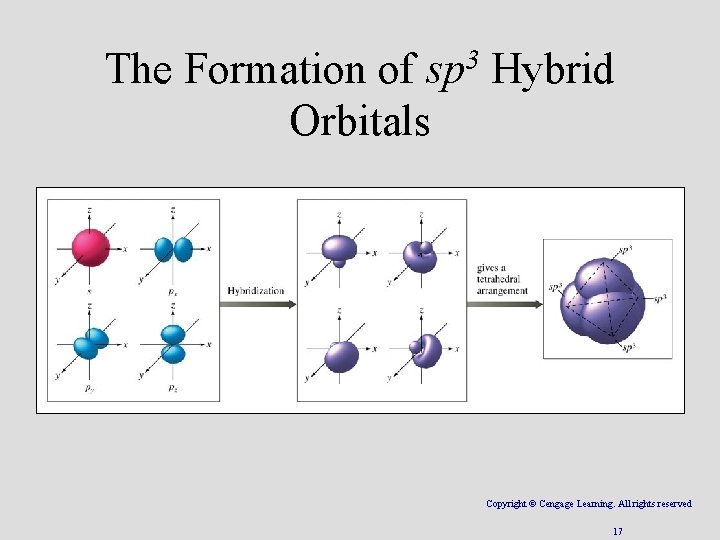
The Formation of sp 3 Hybrid Orbitals Copyright © Cengage Learning. All rights reserved 17

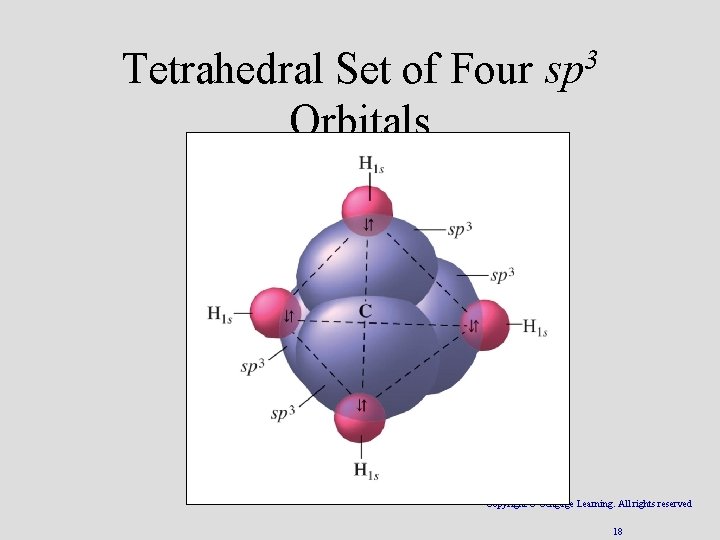
Tetrahedral Set of Four sp 3 Orbitals Copyright © Cengage Learning. All rights reserved 18

2 sp Hybridization • Combination of one s and two p orbitals. • Trigonal Planer – 3 equal attachments. • One p orbital is not used. § Oriented perpendicular to the plane of the sp 2 orbitals. Copyright © Cengage Learning. All rights reserved 19

Sigma ( ) Bond • Electron pair is shared in an area centered on a line running between the atoms. • SINGLE BOND Copyright © Cengage Learning. All rights reserved 20

Pi ( ) Bond • Forms double and triple bonds by sharing electron pair(s) in the space above and below the σ bond. • Uses the unhybridized p orbitals. • DOUBLE OR TRIPLE BOND Copyright © Cengage Learning. All rights reserved 21

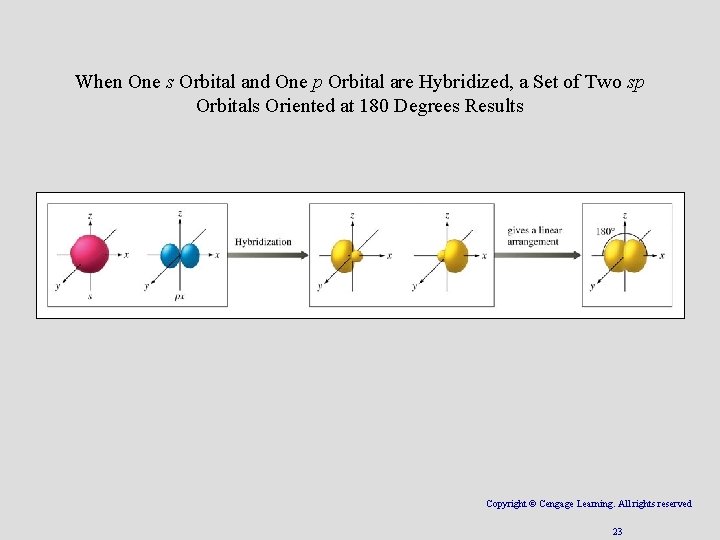
sp Hybridization • Combination of one s and one p orbital. Copyright © Cengage Learning. All rights reserved 22

When One s Orbital and One p Orbital are Hybridized, a Set of Two sp Orbitals Oriented at 180 Degrees Results Copyright © Cengage Learning. All rights reserved 23

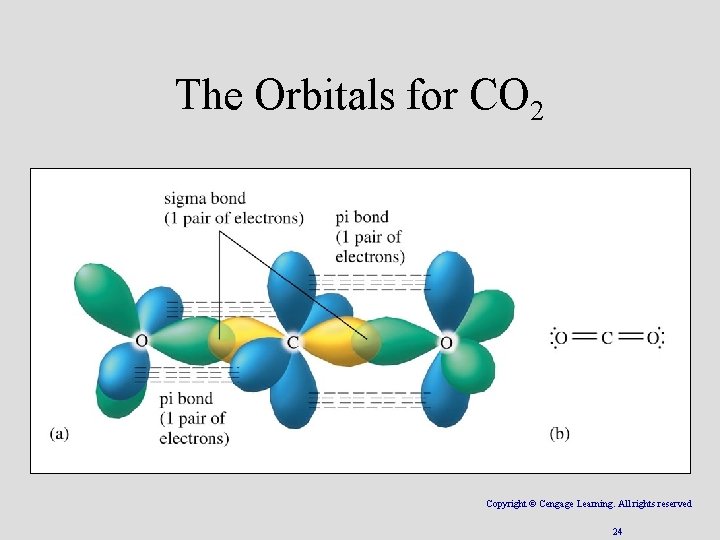
The Orbitals for CO 2 Copyright © Cengage Learning. All rights reserved 24

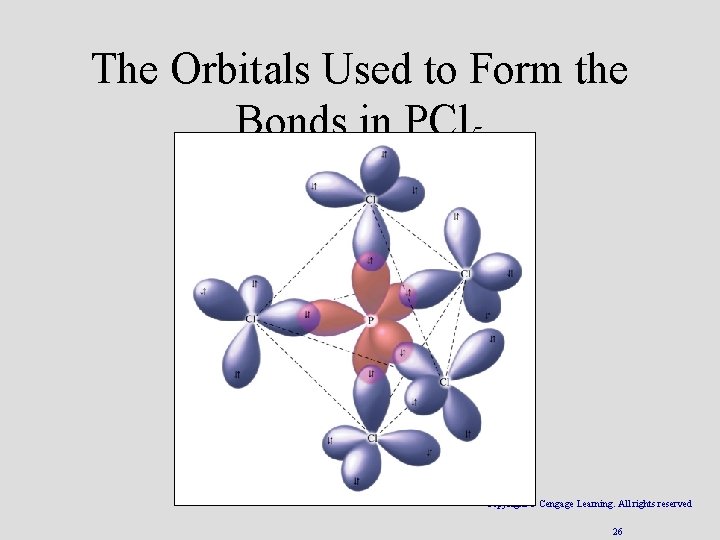
3 sp d Hybridization • Combination of one d, one s, and three p orbitals. • Gives a trigonal bipyramidal arrangement of five equivalent hybrid orbitals. • Recently disproven Copyright © Cengage Learning. All rights reserved 25

The Orbitals Used to Form the Bonds in PCl 5 Copyright © Cengage Learning. All rights reserved 26

3 2 sp d Hybridization • Combination of two d, one s, and three p orbitals. • Gives an octahedral arrangement of six equivalent hybrid orbitals. • Recently disproven Copyright © Cengage Learning. All rights reserved 27

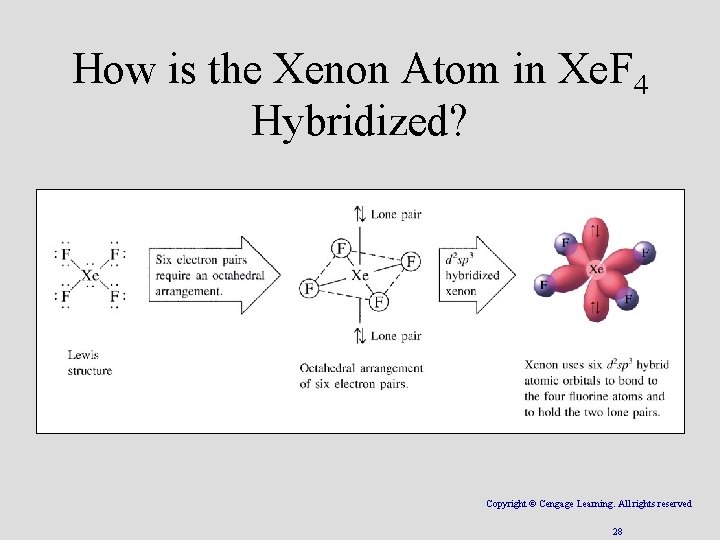
How is the Xenon Atom in Xe. F 4 Hybridized? Copyright © Cengage Learning. All rights reserved 28

Concept Check Draw the Lewis structure for HCN. Which hybrid orbitals are used? Draw HCN: § Showing all bonds between atoms. § Labeling each bond as or . Copyright © Cengage Learning. All rights reserved 29