Valence Electrons n Highest or outermost energy level







- Slides: 19

Valence Electrons n Highest or outermost energy level electrons n n n Where the chemistry happens! Look at principal quantum level n Sulfur: 1 s 22 p 63 s 23 p 4 n n Valence electrons are in n = 3 level Valence electrons = 2 + 4 = 6 electrons

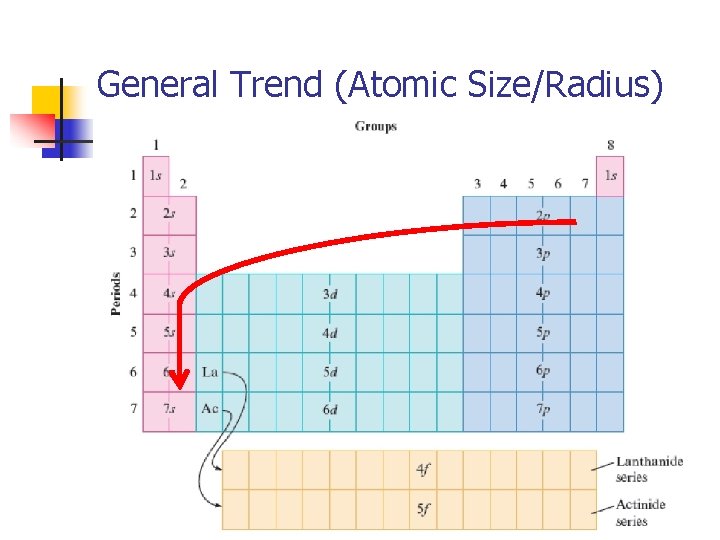
Atomic Radius (Size) n Increases down a group Hydrogen INCREASES! Francium n Orbitals are getting larger and electrons fill further and further from the nucleus at higher energy levels

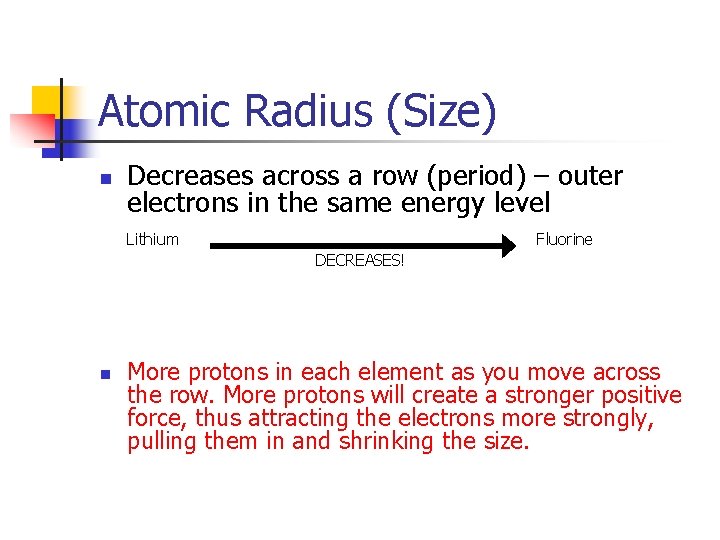
Atomic Radius (Size) n Decreases across a row (period) – outer electrons in the same energy level Lithium Fluorine DECREASES! n More protons in each element as you move across the row. More protons will create a stronger positive force, thus attracting the electrons more strongly, pulling them in and shrinking the size.

Clicker #1 Which of the following elements has the largest atomic radius? A) B) C) D) E) Ca Al N Sr F

General Trend (Atomic Size/Radius)

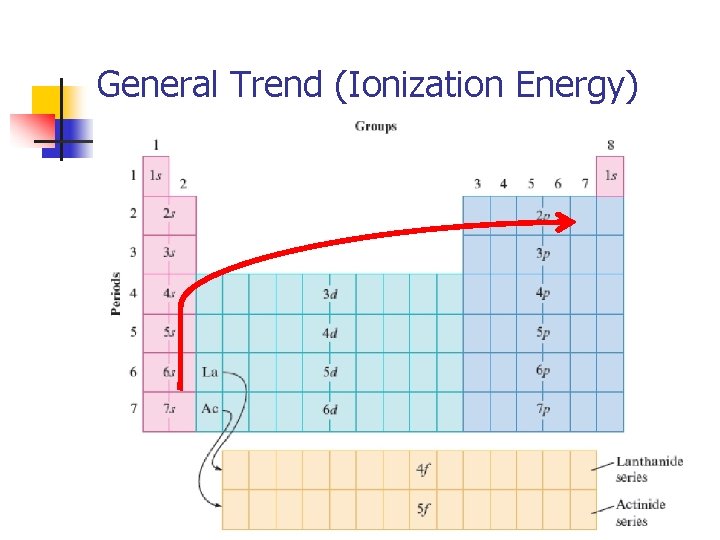
Ionization Energy n Decreases down a group Hydrogen DECREASES! Francium n Orbitals are getting larger and electrons fill further and further from the nucleus at higher energy levels. Electrons are not as tightly bound by the nucleus at higher energy levels so it takes less energy to remove an outer electron.

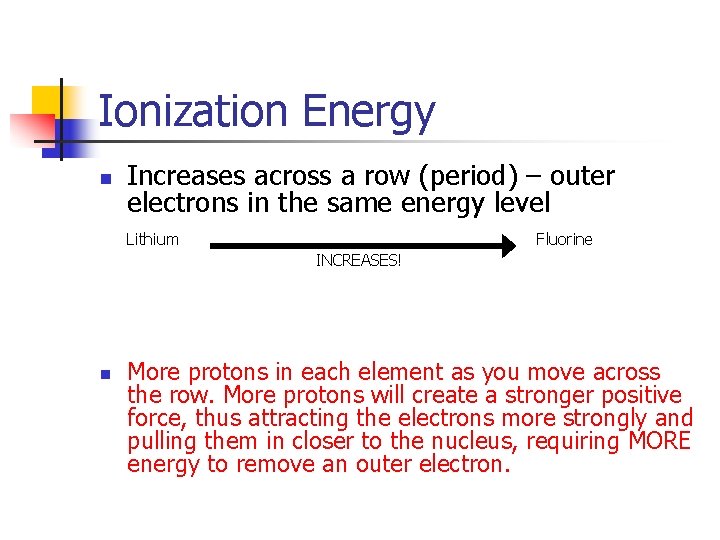
Ionization Energy n Increases across a row (period) – outer electrons in the same energy level Lithium Fluorine INCREASES! n More protons in each element as you move across the row. More protons will create a stronger positive force, thus attracting the electrons more strongly and pulling them in closer to the nucleus, requiring MORE energy to remove an outer electron.

Clicker #2 Which of the following elements has the largest ionization energy? A) B) C) D) E) P Al Cl Ba K

General Trend (Ionization Energy)

Overall goal: Atomic Structure Chemical Bonding 3 D Molecular Structure Molecular Properties

Overall goal: Atomic Structure Chemical Bonding 3 D Molecular Structure Molecular Properties

Bond (Intramolecular) n n Force that holds groups of two or more atoms together and makes them function as a unit. Bond is more stable than the two separated atoms (lower energy state).

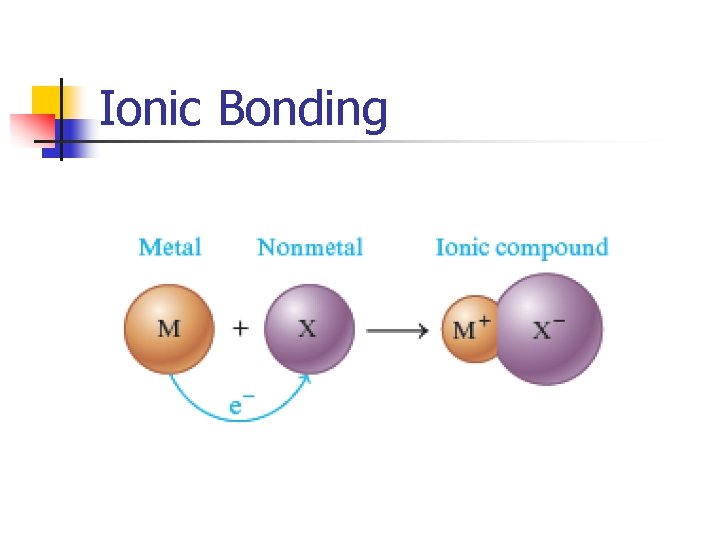
Ionic Bonding

Ionic Bonding

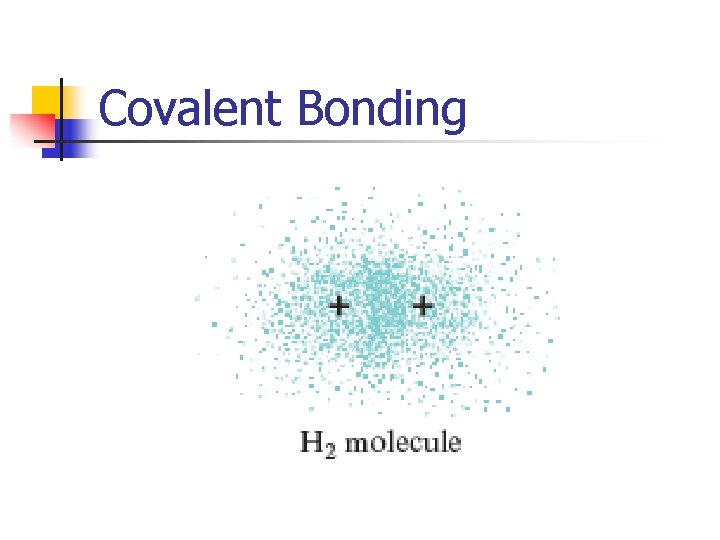
Covalent Bonding

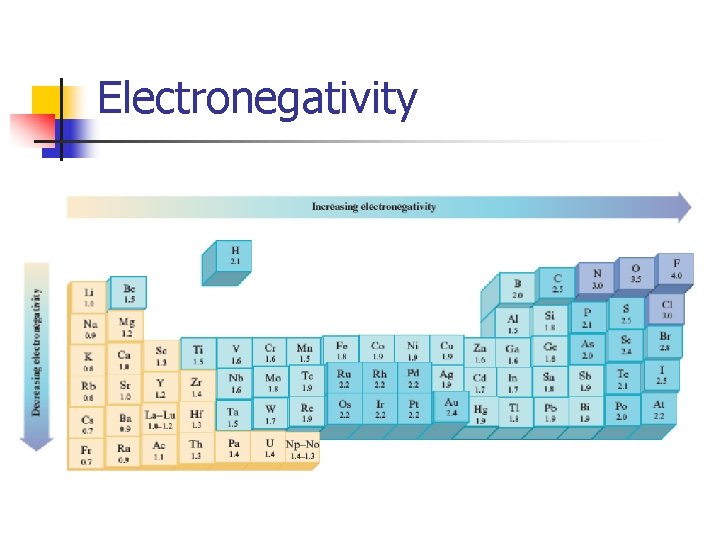
Electronegativity

Covalent Bonding

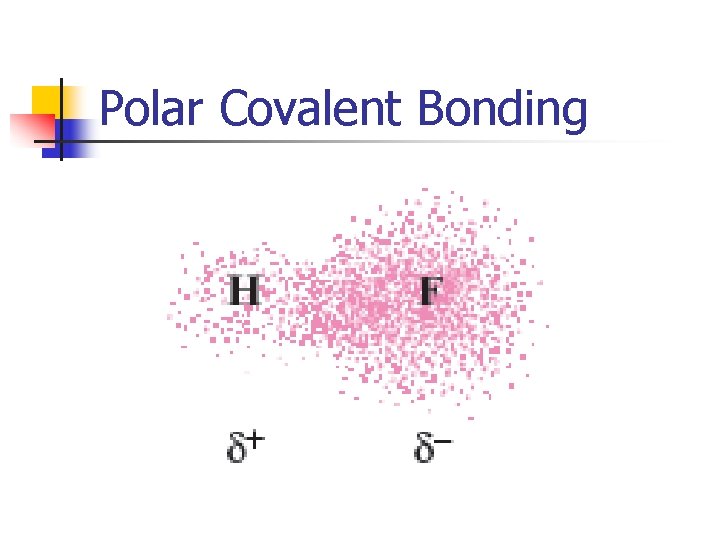
Polar Covalent Bonding

Polar Covalent Bonding