Valence Electrons and Lewis Dot Structures Electron Placement




- Slides: 22

Valence Electrons and Lewis Dot Structures

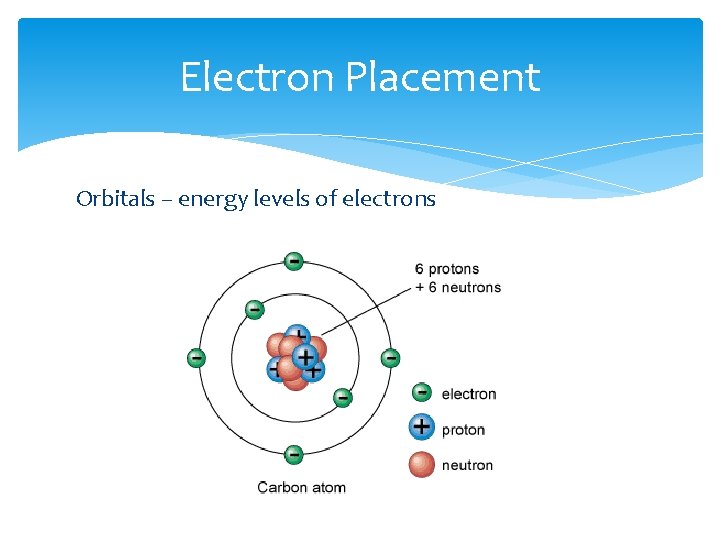
Electron Placement Orbitals – energy levels of electrons

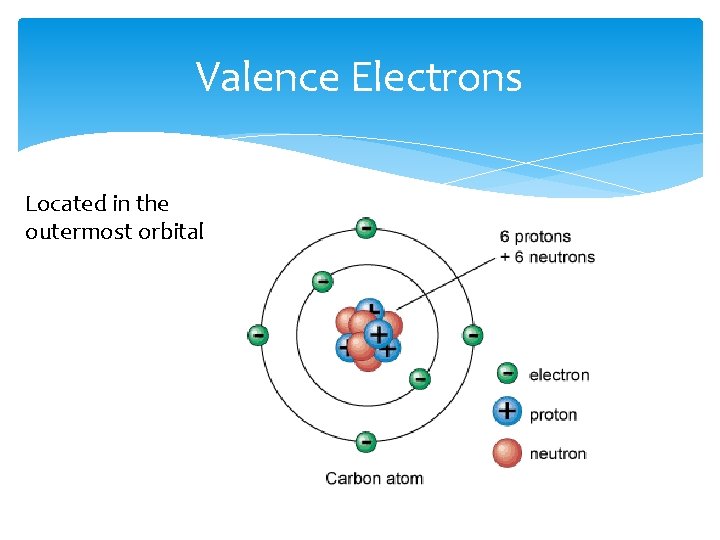
Valence Electrons Located in the outermost orbital

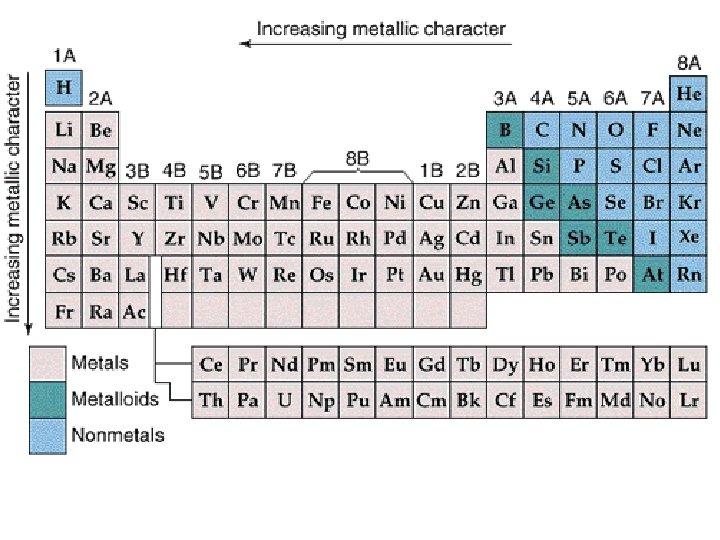
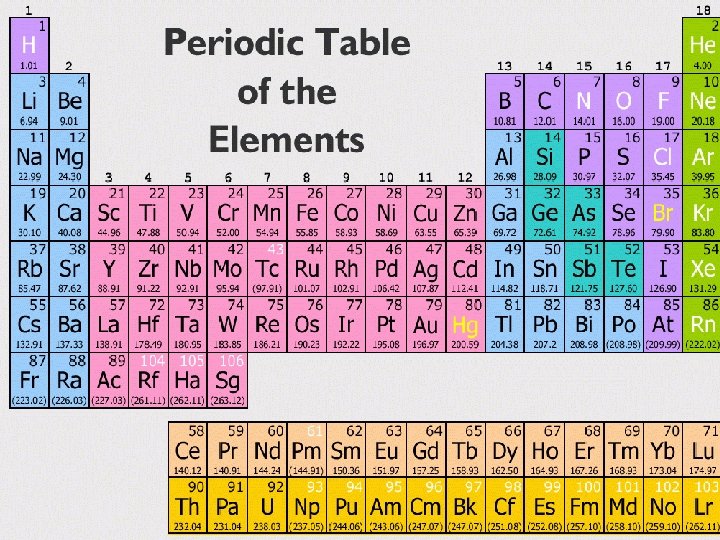
How to determine valence electrons from the periodic table The group number that the element is in tells how many valence electrons it has.


Determining Valence Electrons from the Periodic Table

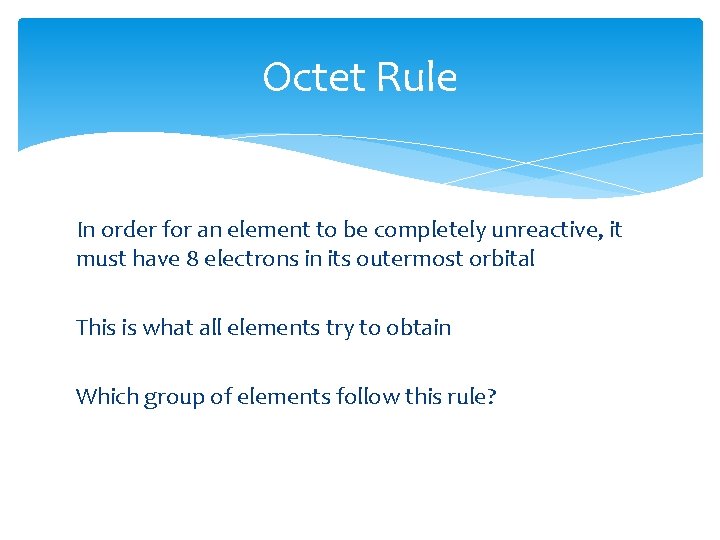
Octet Rule In order for an element to be completely unreactive, it must have 8 electrons in its outermost orbital This is what all elements try to obtain Which group of elements follow this rule?

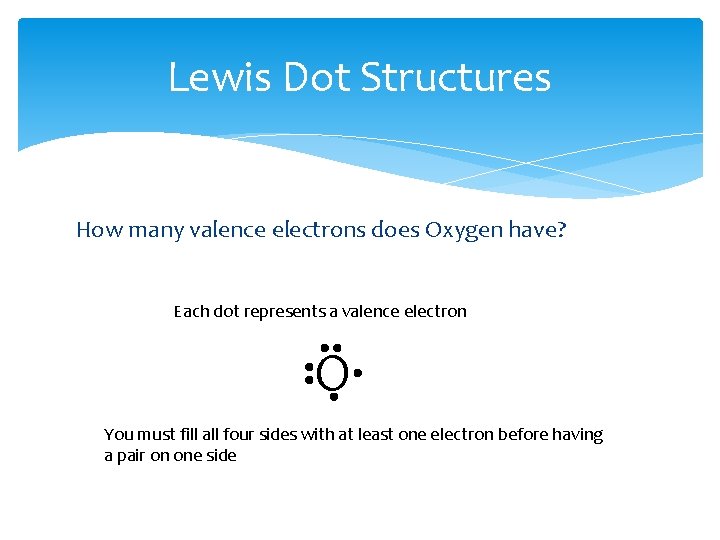
Lewis Dot Structures How many valence electrons does Oxygen have? Each dot represents a valence electron O You must fill all four sides with at least one electron before having a pair on one side

Lewis Dot Structures How many valence electrons does Nitrogen have? N

Lewis Dot Structure Helium is the only exception. Helium only has 2 electrons total. Although it does not follow the octet rule, its outer shell is full, and is therefore stable. He

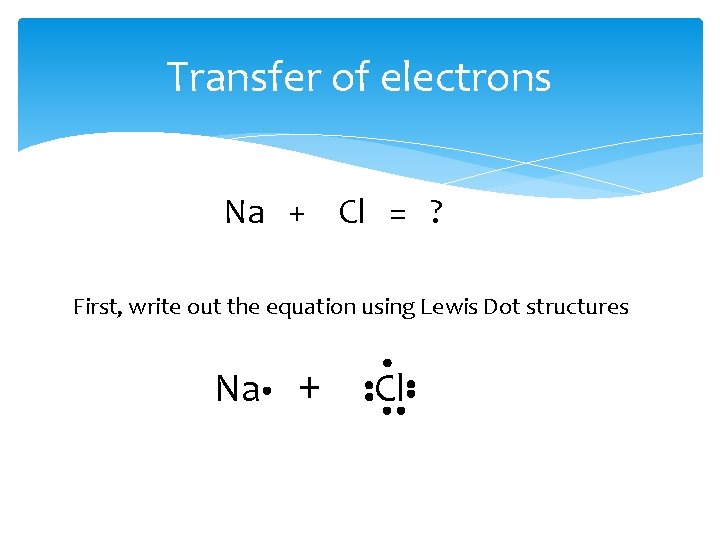
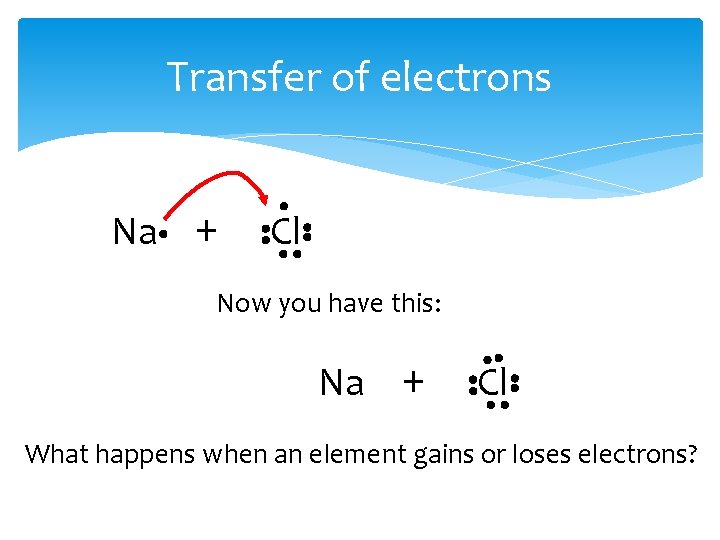
Transfer of electrons Na + Cl = ? First, write out the equation using Lewis Dot structures Na + Cl

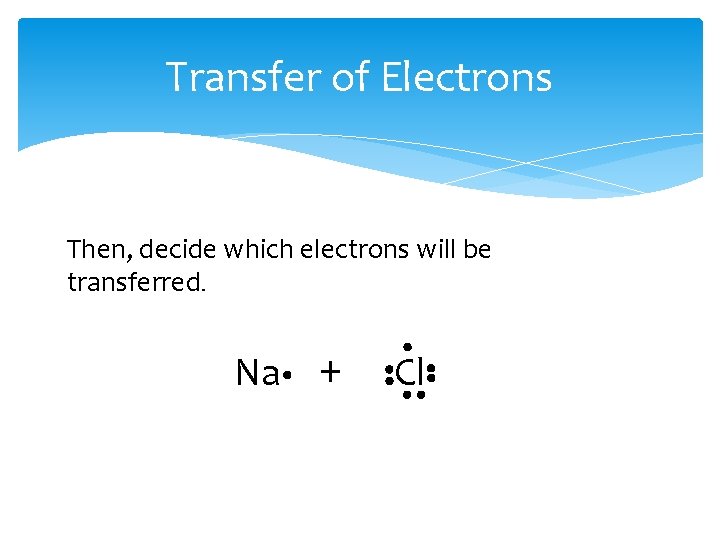
Transfer of Electrons Then, decide which electrons will be transferred. Na + Cl

Transfer of electrons Na + Cl Now you have this: Na + Cl What happens when an element gains or loses electrons?

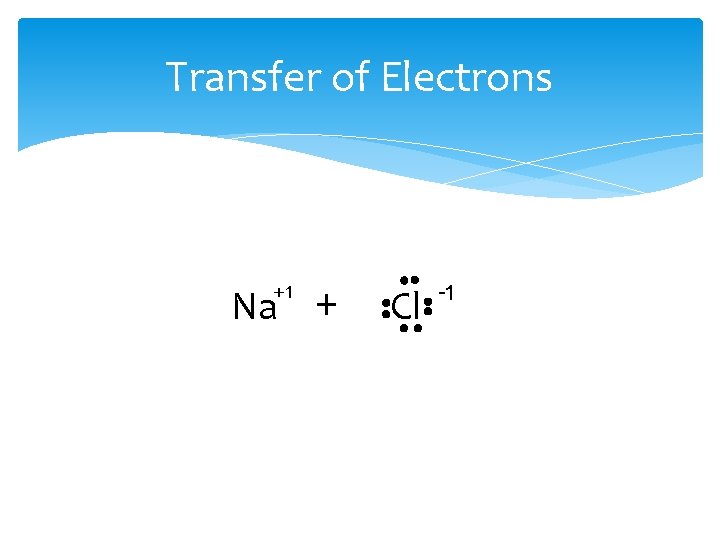
Transfer of Electrons +1 Na + Cl -1

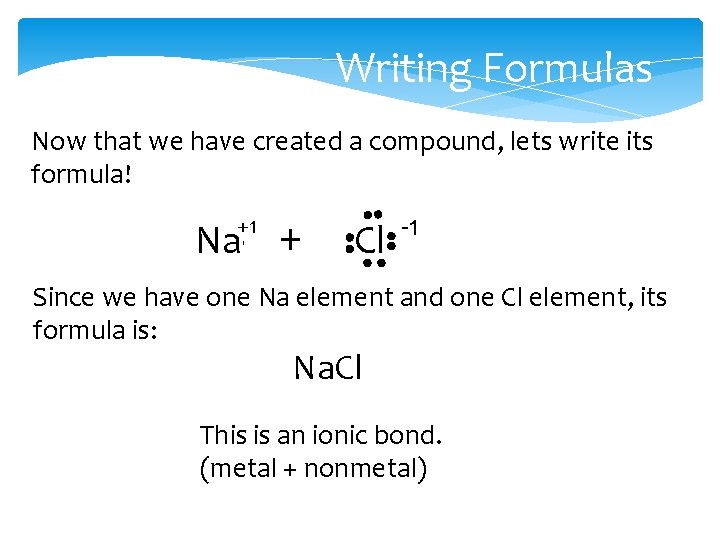
Writing Formulas Now that we have created a compound, lets write its formula! +1 Na + Cl -1 Since we have one Na element and one Cl element, its formula is: Na. Cl This is an ionic bond. (metal + nonmetal)

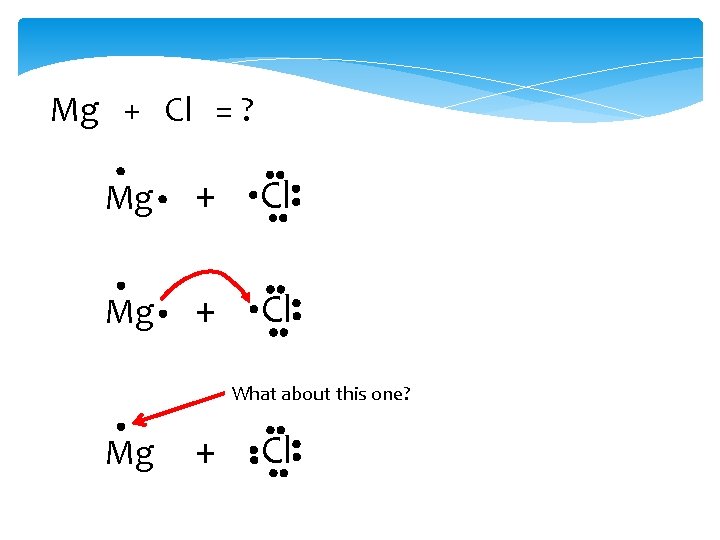
Mg + Cl = ? Mg + Cl What about this one? Mg + Cl

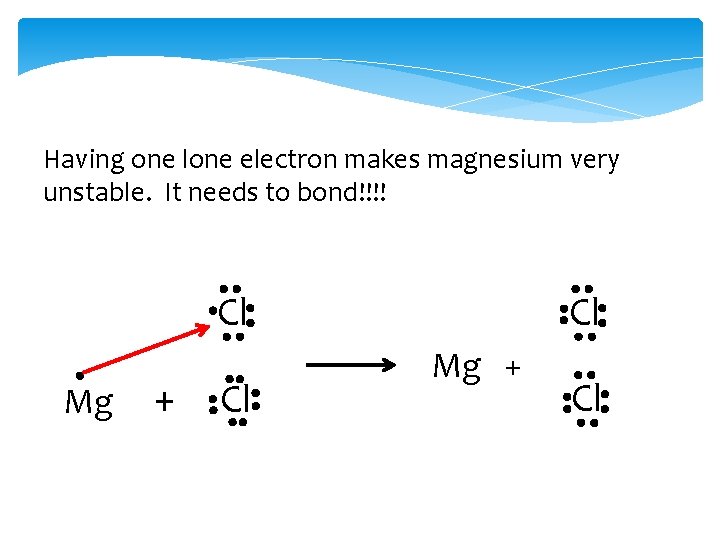
Having one lone electron makes magnesium very unstable. It needs to bond!!!! Cl Cl Mg + Cl

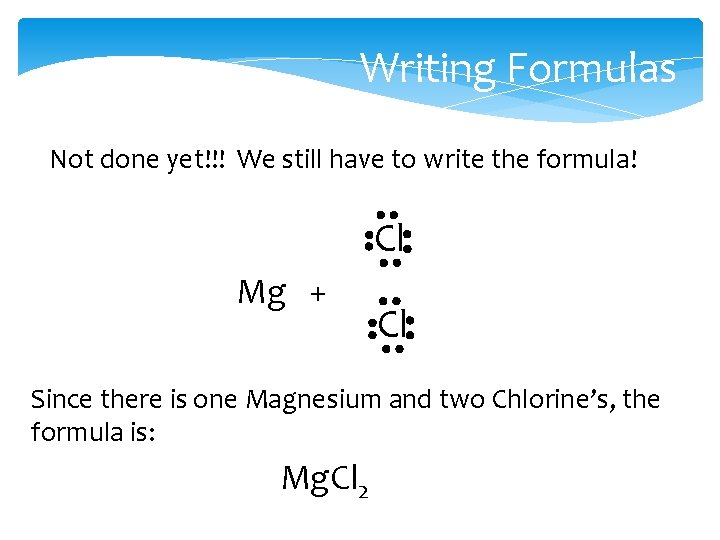
Writing Formulas Not done yet!!! We still have to write the formula! Cl Mg + Cl Since there is one Magnesium and two Chlorine’s, the formula is: Mg. Cl 2

Covalent Bonds Covalent bonds do NOT transfer electrons, they share them Covalent bonds exist between two nonmetals (nonmetal + nonmetal)

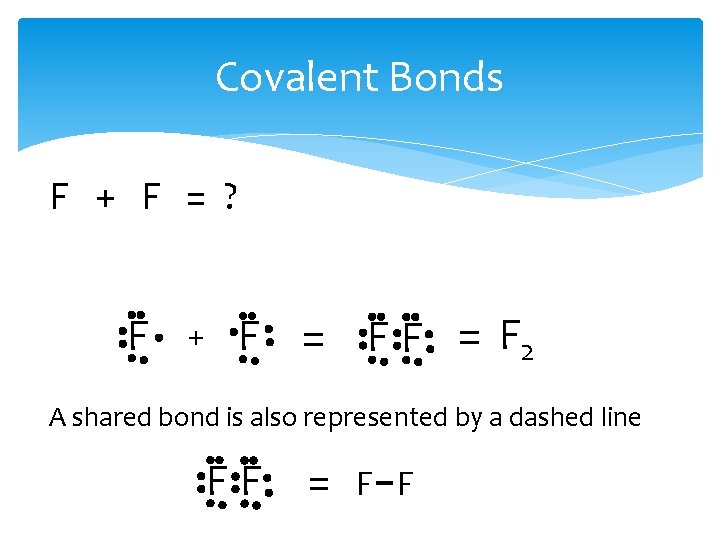
Covalent Bonds F + F = ? F + F = F 2 A shared bond is also represented by a dashed line FF = F F

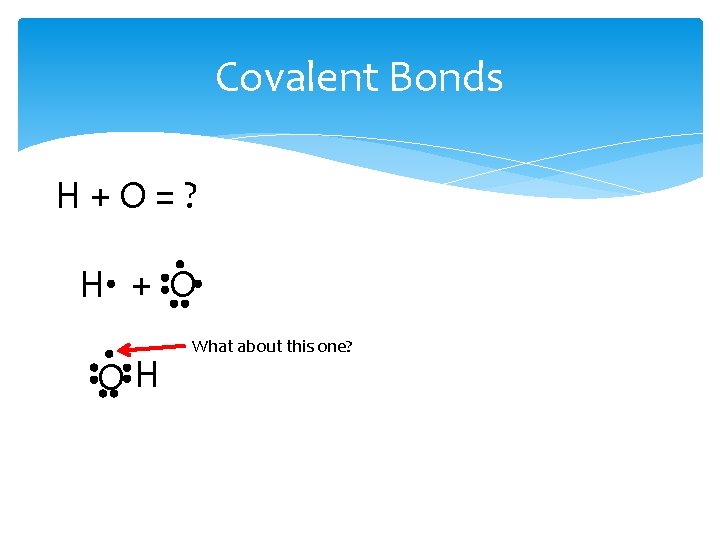
Covalent Bonds H+O=? H + O OH What about this one?

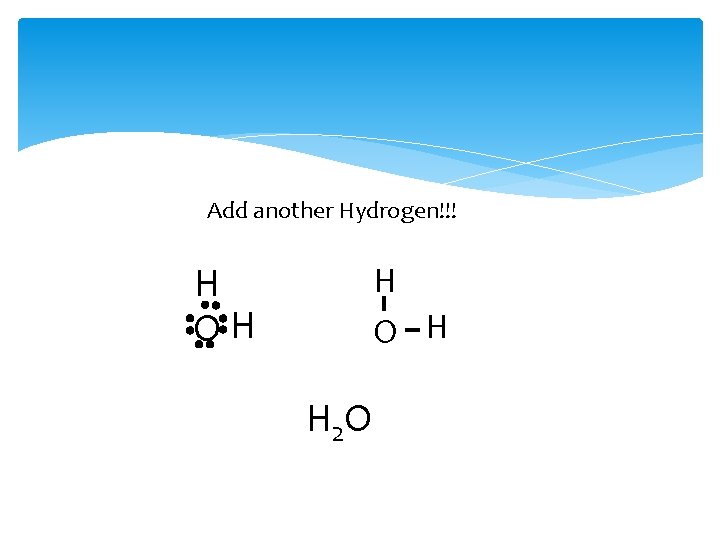
Add another Hydrogen!!! H O H H OH H 2 O