Valence Electrons Activity Fold your periodic table so

Valence Electrons

Activity • Fold your periodic table so that the f and d blocks are looped. • Determine the number of valence electrons for each remaining group (column). • Find the pattern.

Valence Electrons • Valence electrons – Electrons in an atom’s highestnumbered energy level. • e- config. for Si: 1 s 2 2 p 6 3 s 2 3 p 2 • Si has four valence electrons. • 3 s 2 + 3 p 2 = 4 e • e- config. for C: 1 s 2 2 p 2 • C also has four valence electrons.

Valence Electrons • How many valence electrons does selenium have (element 34)? • e- config: 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 4 • Se has 6 valence electrons.
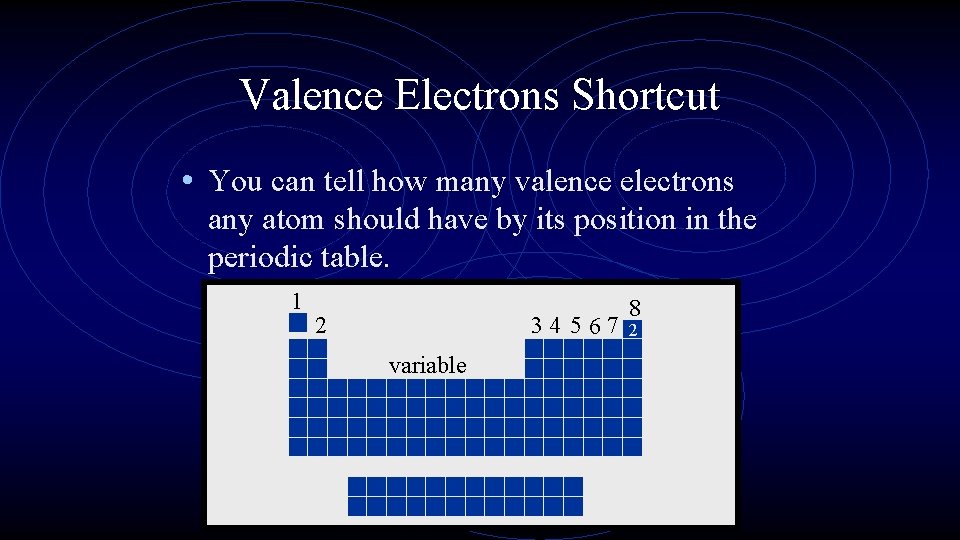
Valence Electrons Shortcut • You can tell how many valence electrons any atom should have by its position in the periodic table. 1 2 34 5 67 variable 8 2

Why are the valence electrons responsible for bonding?

How many valence electrons are in each of the following elements: Na (sodium) Mg (Magnesium) Xe (Xenon) Br (Bromine) C (Carbon) F (Fluorine) O (Oxygen) K (Potassium)
Draw a picture to explain why the valence electrons are responsible for bonding between different elements. Try to use the s & p orbital shapes.

Octet Rule


How many valence electrons do each of the following elements want to lose or gain? Na (sodium) Mg (Magnesium) Xe (Xenon) Br (Bromine) C (Carbon) F (Fluorine) O (Oxygen) K (Potassium)

Now, estimate the reactivity of each of the following elements based on the valence electrons. Na (sodium) Mg (Magnesium) Xe (Xenon) Br (Bromine) C (Carbon) F (Fluorine) O (Oxygen) K (Potassium)

Lewis Dot Diagrams

Lewis Dot Diagrams • Electron Dot Diagram – shows the valence electrons of an atom as dots. • Distribute dots around atomic symbol to represent valence electrons. • Should never have more than 8 dots.
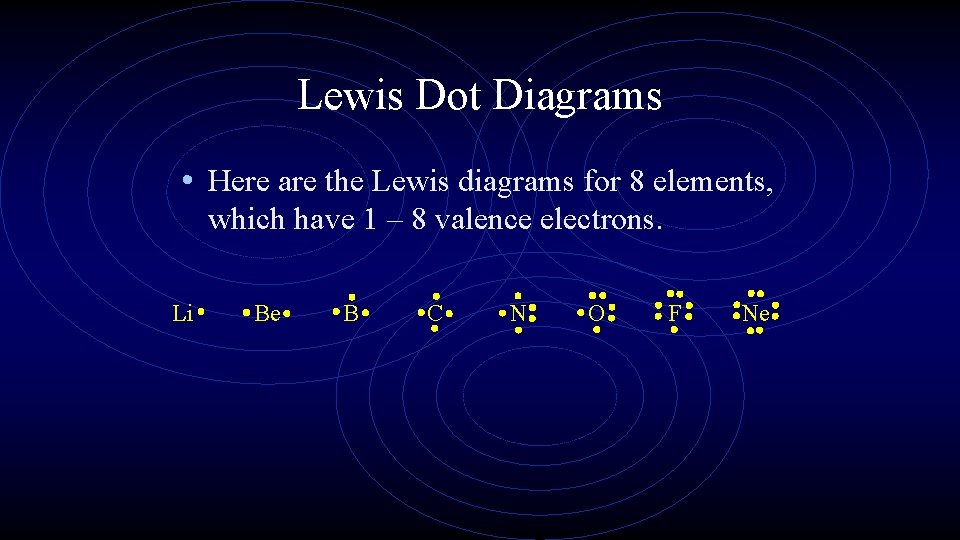
Lewis Dot Diagrams • Here are the Lewis diagrams for 8 elements, which have 1 – 8 valence electrons. Li Be B C N O F Ne
Draw the Lewis Dot structure for each of the following elements: Na (sodium) Mg (Magnesium) Xe (Xenon) Br (Bromine) C (Carbon) F (Fluorine) O (Oxygen) K (Potassium)
Periodic Trends

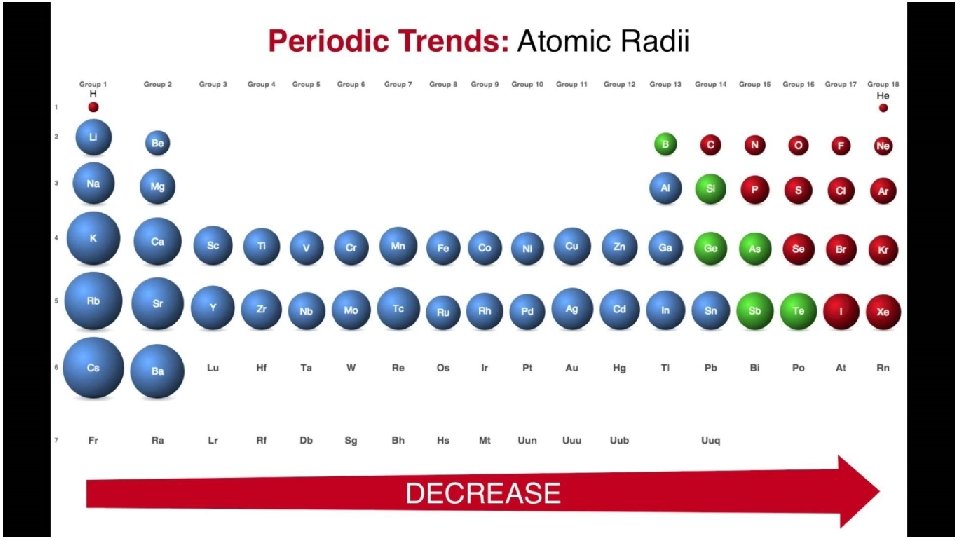
Trends • Atomic radius • Electronegativity • Ionization Energy • How are all these important for bonding to form molecules?
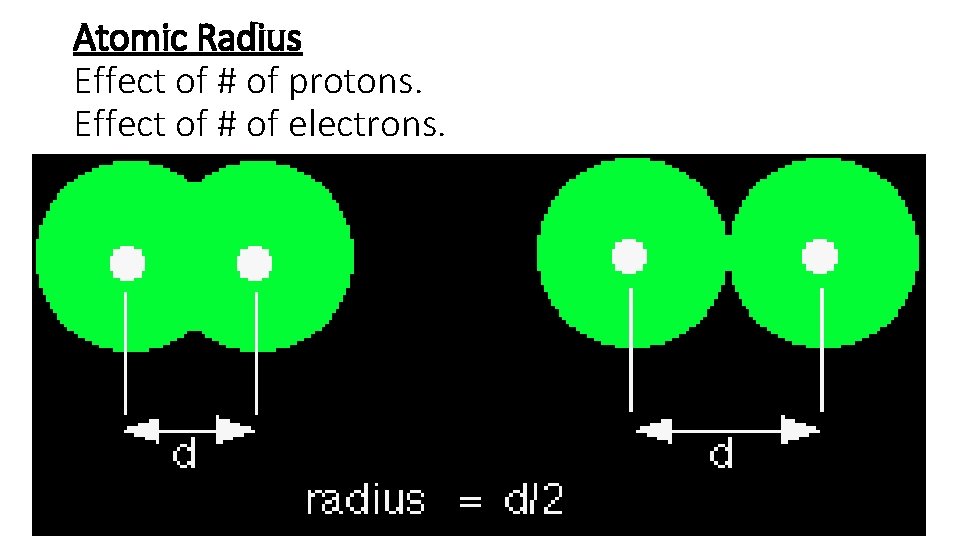
Atomic Radius Effect of # of protons. Effect of # of electrons.
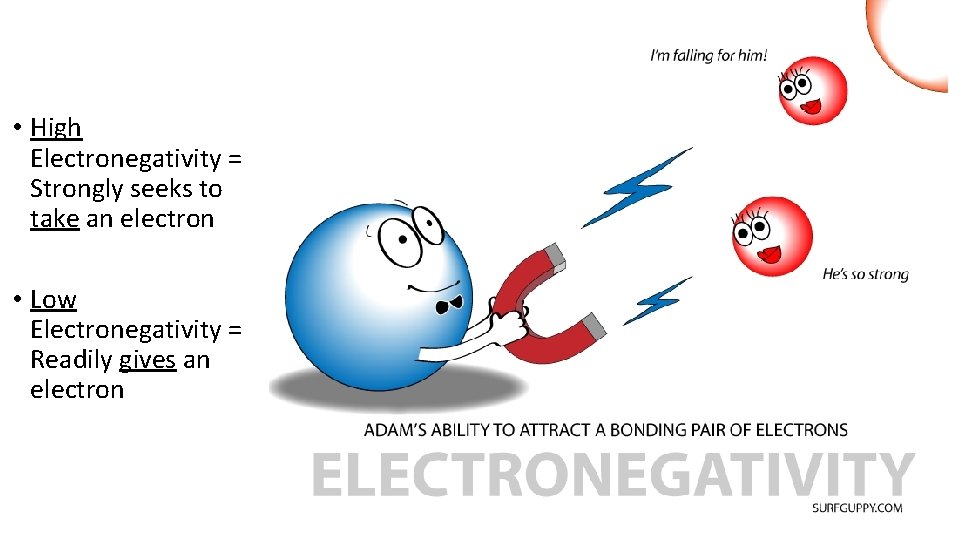
• High Electronegativity = Strongly seeks to take an electron • Low Electronegativity = Readily gives an electron
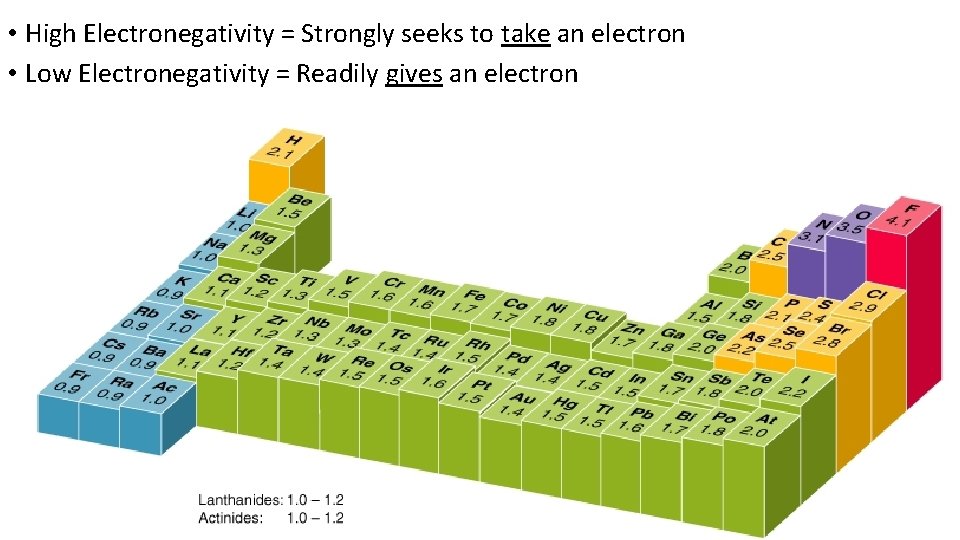
• High Electronegativity = Strongly seeks to take an electron • Low Electronegativity = Readily gives an electron
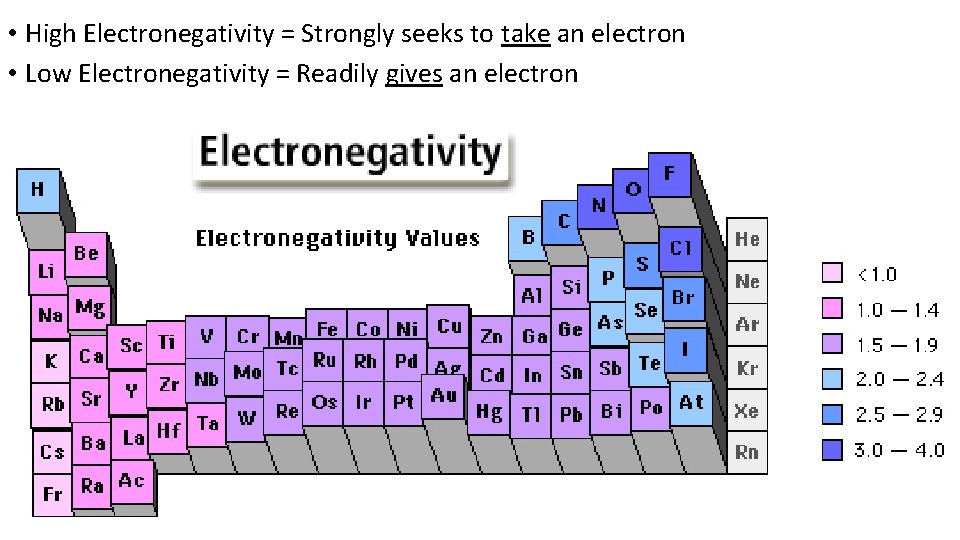
• High Electronegativity = Strongly seeks to take an electron • Low Electronegativity = Readily gives an electron
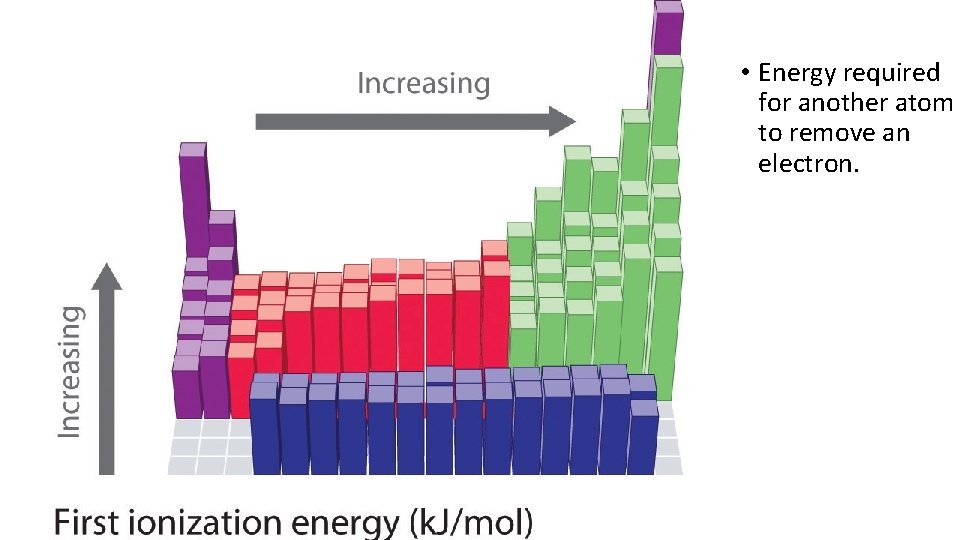
• Energy required for another atom to remove an electron.
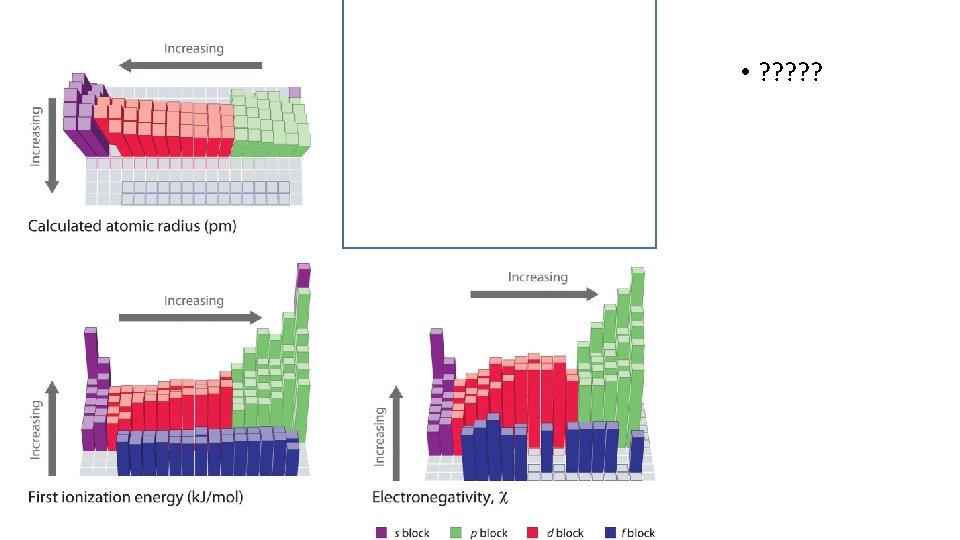
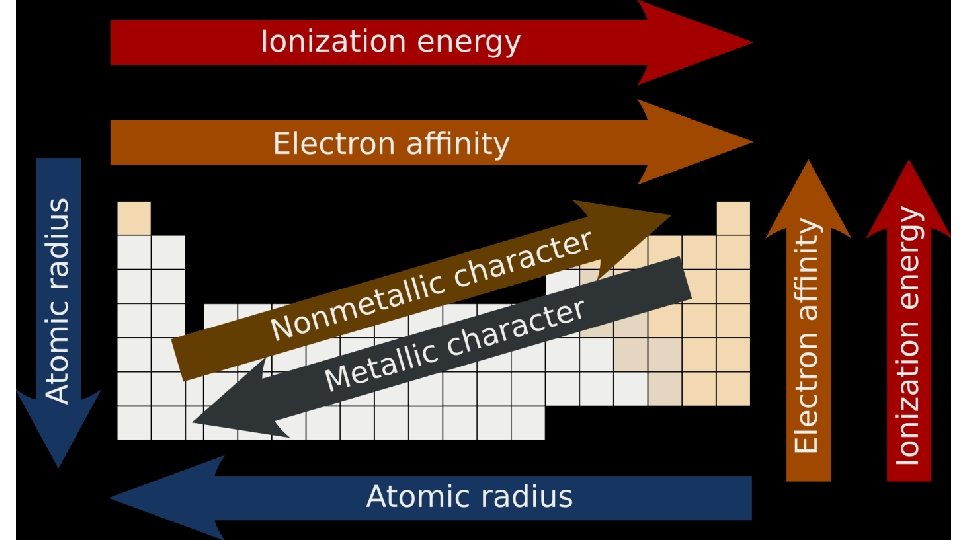

Trends • Atomic radius • How is it measured • Electronegativity • Ionization Energy • Melting Point • Boiling Point • Conductivity • How are all these important for bonding to form molecules?

Predict which elements will react with other elements.
- Slides: 28