VALENCE BOND THEORY MOLECULAR ORBITAL THEORY Chapter 3
VALENCE BOND THEORY & MOLECULAR ORBITAL THEORY Chapter 3 Dr. Shuchita Agrawal Government P. G. College, Damoh 1

Valence Bond Theory • Extends the Lewis concept of electron pair bonds by introducing the notion of orbital overlap and hybridization. • Hybridization is necessary to account for molecular geometry predicted by VSEPR. • Hybrid atomic orbitals are constructed by making linear combinations (sums and differences) between hydrogen-like orbitals in the valence shell of each atom. 2
Hybridization • Example: Be. H 2 • Linear Molecule, equivalent Be-H bonds • Symmetry Adapted Linear Combination (SALC) of hydrogen-like atomic orbitals in the valence shell of Be • Basis set consists of: 2 s, 2 px, 2 py, 2 pz • Only 2 s and 2 px are mixed; 2 py and 2 pz retain their original form. • “ideal” hybrids ½ s, ½ p character • Energetic “cost” of hybridization (promotion energy) is offset by formation of stronger bonds 3
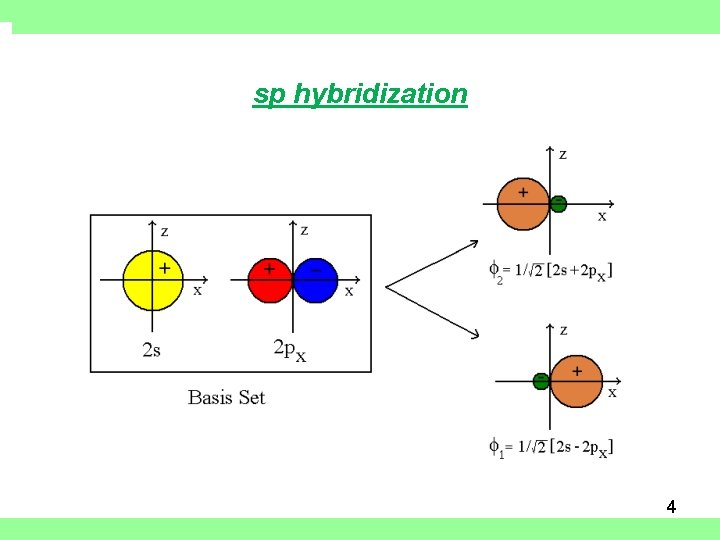
sp hybridization 4

Bonding Description • Valence electrons of each atom are distributed among the available orbitals. • In this case, the two valence electrons of Be are placed in the sp hybrid orbitals; the remaining p orbitals are vacant. • Each H atom has one electron in the 1 s orbital. • Orbitals of each atom overlap to form electron pair bonds. 5
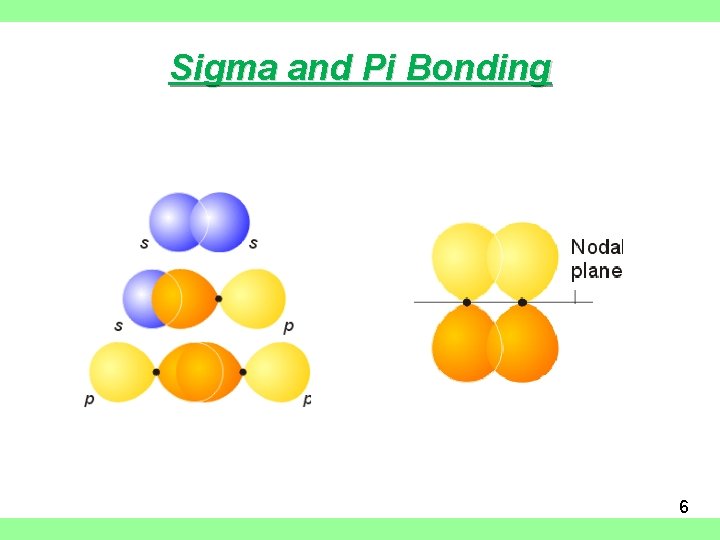
Sigma and Pi Bonding 6
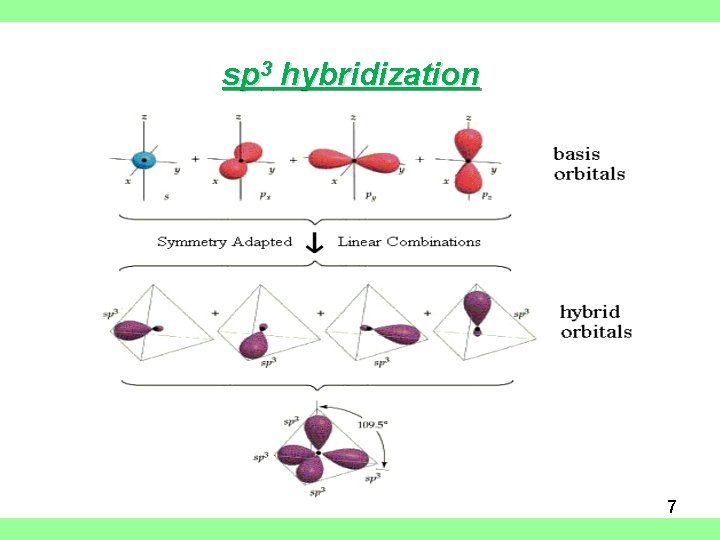
sp 3 hybridization 7

VBT description of H 2 O • • Oxygen has sp 3 hybridization. Ideal tetrahedral angle is 109. 5 o Observed bond angle in H 2 O is ~ 105 o This indicates that hybrids involved in O-H bonding orbitals have more pcharacter. • Lone-pair orbitals on oxygen have more s-character. 8
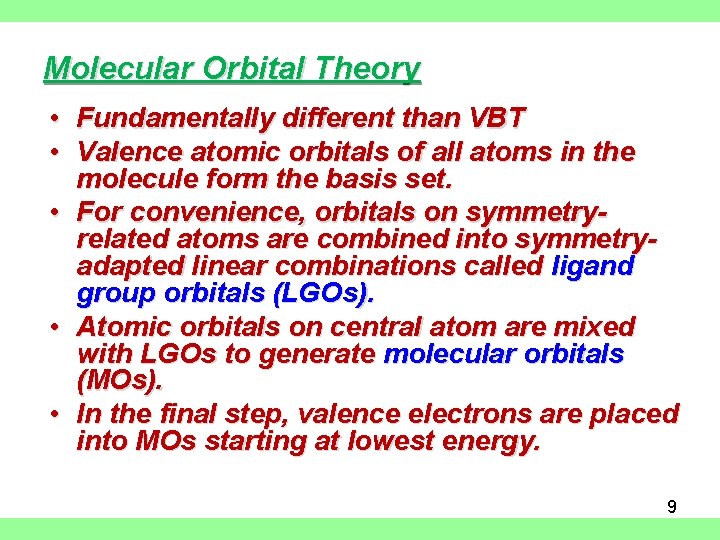
Molecular Orbital Theory • Fundamentally different than VBT • Valence atomic orbitals of all atoms in the molecule form the basis set. • For convenience, orbitals on symmetryrelated atoms are combined into symmetryadapted linear combinations called ligand group orbitals (LGOs). • Atomic orbitals on central atom are mixed with LGOs to generate molecular orbitals (MOs). • In the final step, valence electrons are placed into MOs starting at lowest energy. 9
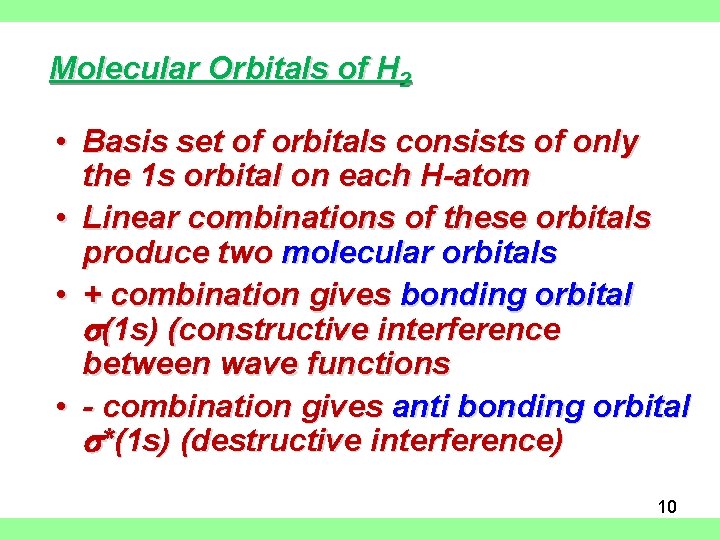
Molecular Orbitals of H 2 • Basis set of orbitals consists of only the 1 s orbital on each H-atom • Linear combinations of these orbitals produce two molecular orbitals • + combination gives bonding orbital s(1 s) (constructive interference between wave functions • - combination gives anti bonding orbital s*(1 s) (destructive interference) 10
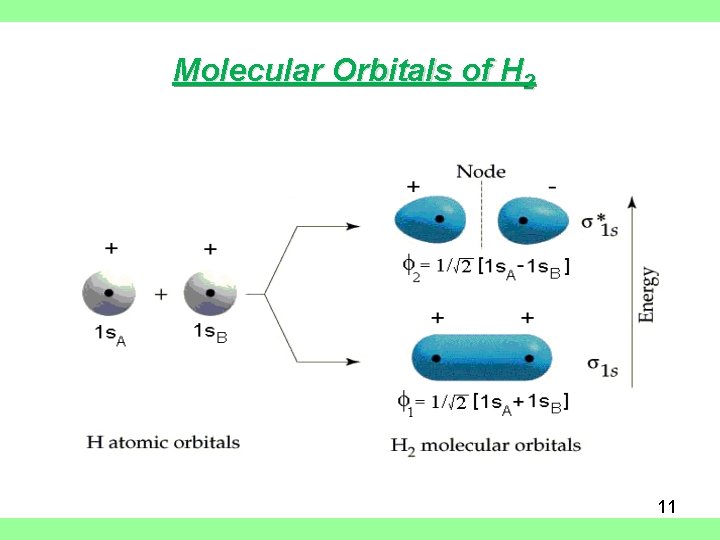
Molecular Orbitals of H 2 11
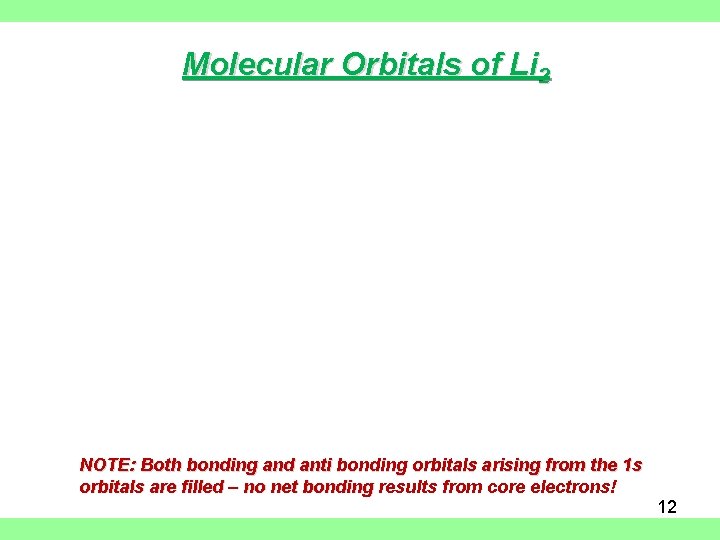
Molecular Orbitals of Li 2 NOTE: Both bonding and anti bonding orbitals arising from the 1 s orbitals are filled – no net bonding results from core electrons! 12

p-Orbital overlap modes 13

Homo nuclear Diatomic with p-Orbitals 14
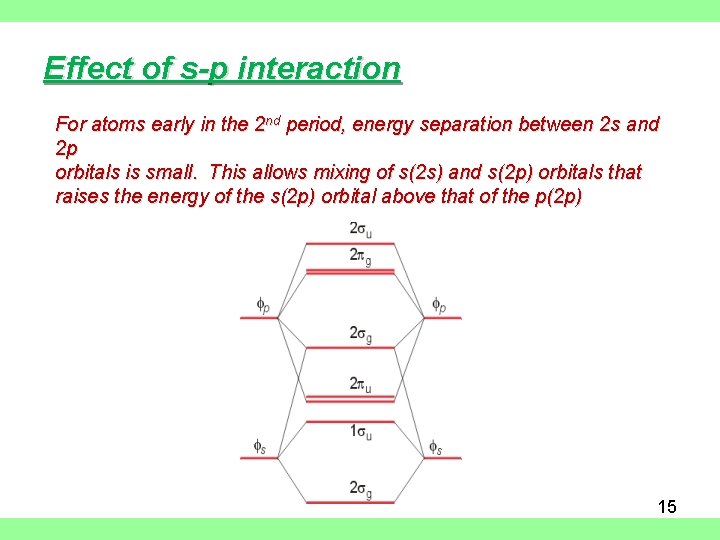
Effect of s-p interaction For atoms early in the 2 nd period, energy separation between 2 s and 2 p orbitals is small. This allows mixing of s(2 s) and s(2 p) orbitals that raises the energy of the s(2 p) orbital above that of the p(2 p) 15
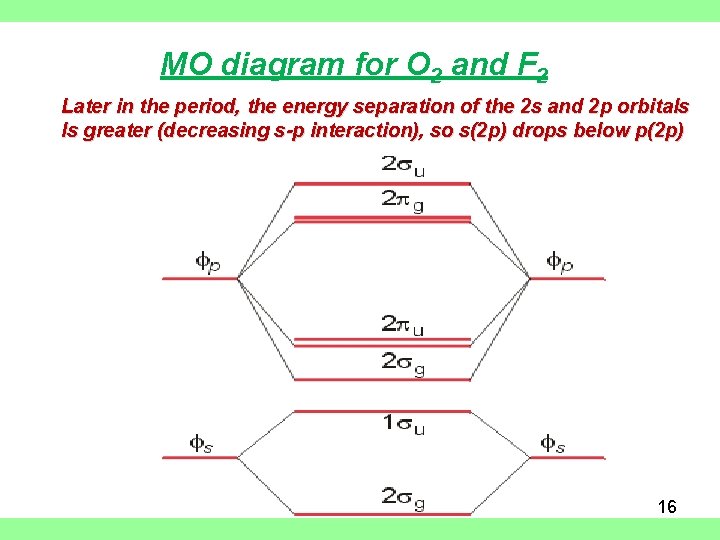
MO diagram for O 2 and F 2 Later in the period, the energy separation of the 2 s and 2 p orbitals Is greater (decreasing s-p interaction), so s(2 p) drops below p(2 p) 16
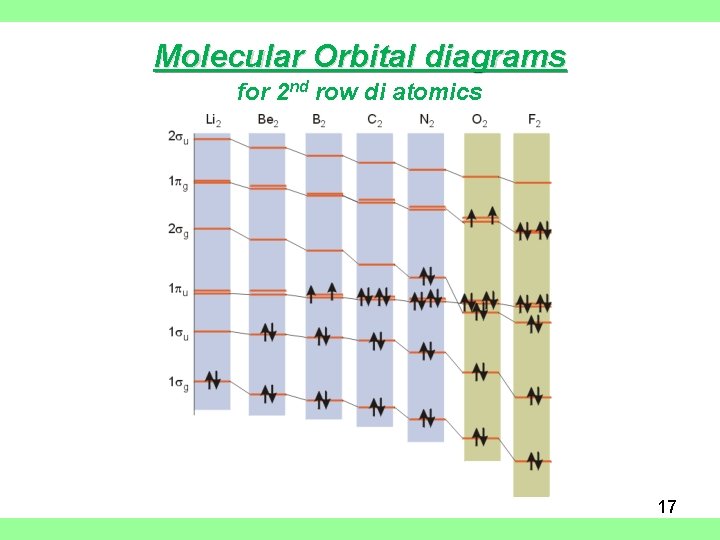
Molecular Orbital diagrams for 2 nd row di atomics 17
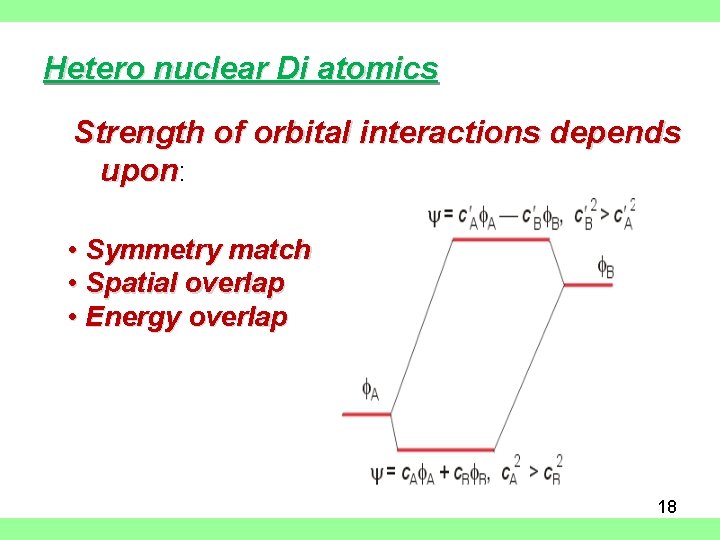
Hetero nuclear Di atomics Strength of orbital interactions depends upon: • Symmetry match • Spatial overlap • Energy overlap 18
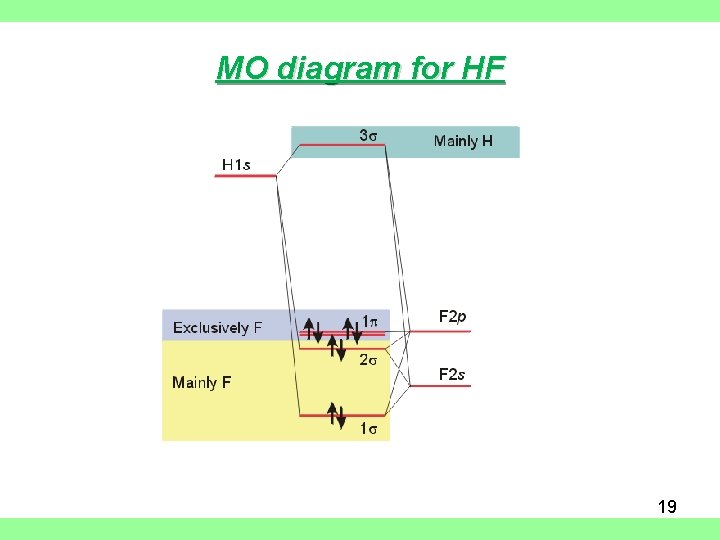
MO diagram for HF 19
Bonding in H 2 O 20
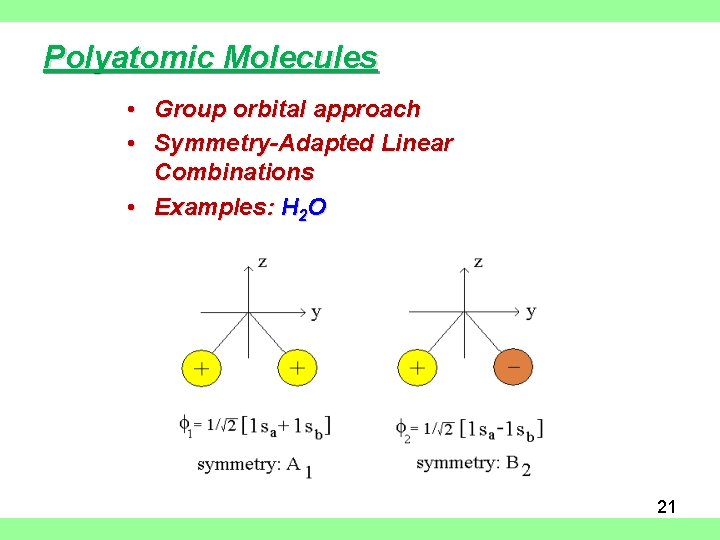
Polyatomic Molecules • Group orbital approach • Symmetry-Adapted Linear Combinations • Examples: H 2 O 21
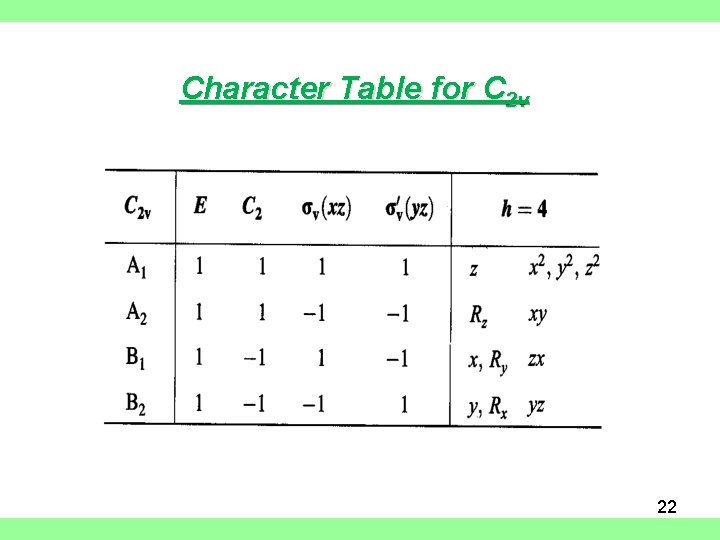
Character Table for C 2 v 22
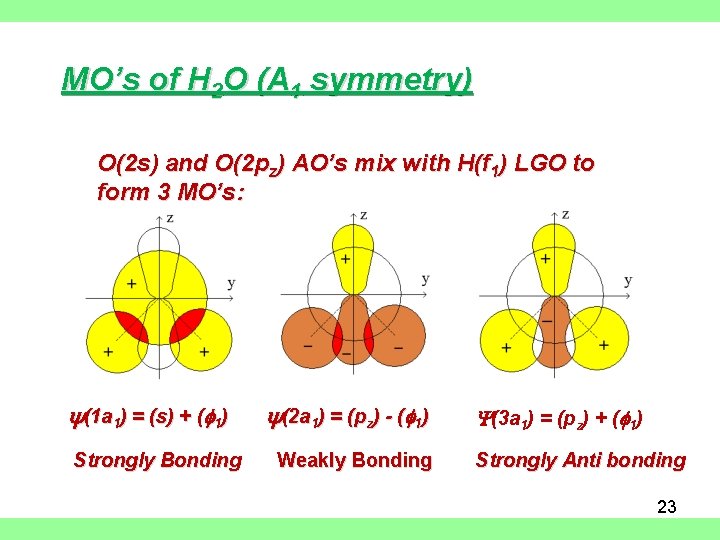
MO’s of H 2 O (A 1 symmetry) O(2 s) and O(2 pz) AO’s mix with H(f 1) LGO to form 3 MO’s: y(1 a 1) = (s) + (f 1) Strongly Bonding y(2 a 1) = (pz) - (f 1) Weakly Bonding Y(3 a 1) = (pz) + (f 1) Strongly Anti bonding 23
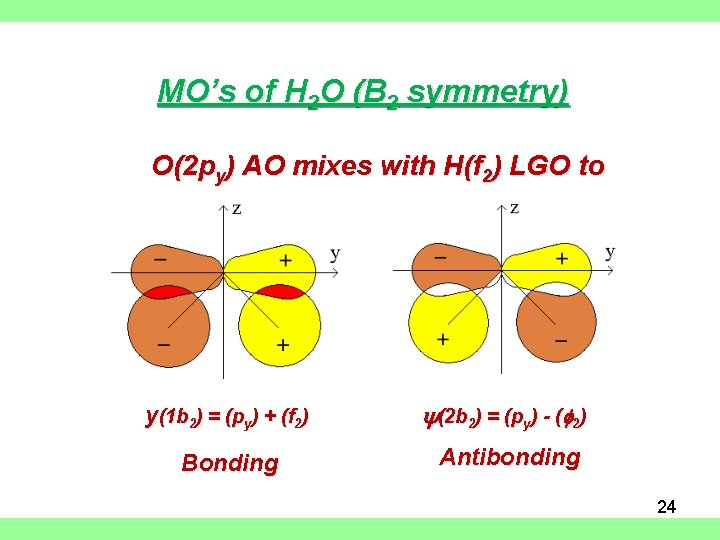
MO’s of H 2 O (B 2 symmetry) O(2 py) AO mixes with H(f 2) LGO to form 2 MO’s: y(1 b 2) = (py) + (f 2) y(2 b 2) = (py) - (f 2) Bonding Antibonding 24
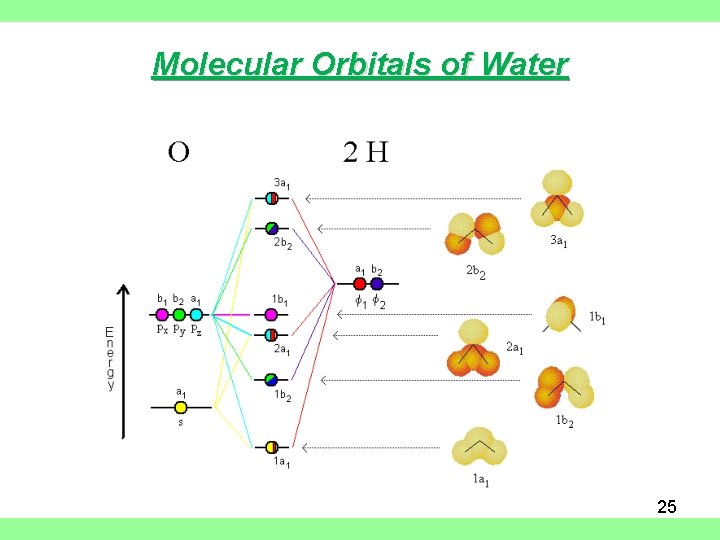
Molecular Orbitals of Water 25
Bonding in SF 6 26
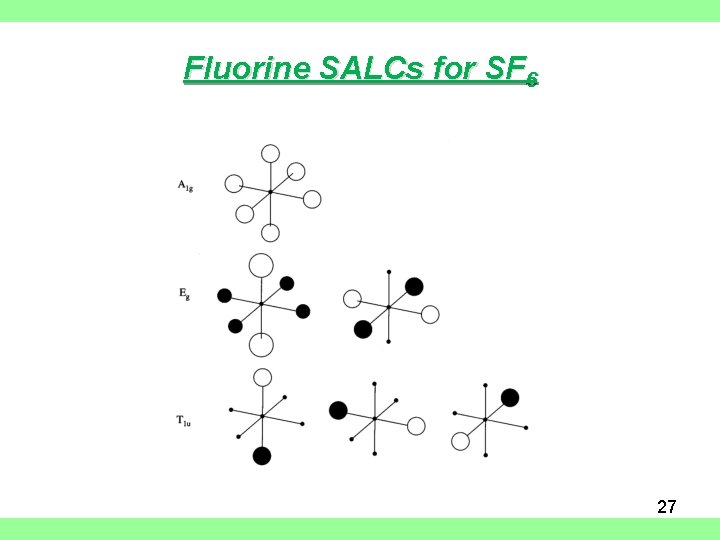
Fluorine SALCs for SF 6 27

MO diagram for SF 6 28

Thanks • EMAIL ID: mrs. shuchitaagrawal@gmail. com • Ph No : 9977330500 Dr. Shuchita Agrawal Government P. G. College, Damoh 29
- Slides: 29