Vaccination of Adolescents New Frontiers Andrew Kroger National












































- Slides: 51

Vaccination of Adolescents: New Frontiers Andrew Kroger National Center for Immunization and Respiratory Diseases National Assembly on Schoolbased Health Care (NASBHC) May 16, 2007

Vaccine Research and Development Basic research, animal studies Phase I: safety, Immunogenicity(10 -20) Phase II: Dose ranging (20 several hundred) Phase III: safety, efficacy (several hundred to several thousand) Biologics License Application

Vaccines of the Future Cytomegalovirus Human immunodeficiency virus Herpes simplex virus Group B streptococcus

Challenges to New Vaccine Development Understanding the immune response to natural infection not complete Humoral and cell-based immune response important to developing an effective vaccine Need to be able to test efficacy – often outcomes difficult to study due to long latency

Cytomegalovirus Herpesvirus Latent virus with reactivation Infection is Common Congenital CMV Severe in patients with Altered Immunocompetence

CMV Vaccines Candidates • Live-attenuated and subunit vaccines (Phase I) • Vector vaccines (canarypox virus vector) Challenges • Humoral and cellular response critical

Human Immunodeficiency Virus (HIV) Infection 1 million infected in U. S. 25% unaware of their infection Progresses to Acquired Immunodeficiency Syndrome (AIDS) HIV virus transmission – sexual and percutaneous HIV pathogenesis percutaneous and mucosal route

HIV Vaccine Trials Network 56 trials occurring in 25 sites worldwide 29 candidate vaccines Strategies • Recombinant vectors • Prime-boost approach

HIV Vaccines Challenges • Effective animal models for preclinical trials • Determining appropriate outcomes to measure

Herpes simplex virus (HSV) Family Herpesvirus Reactivation disease HSV 1: Cold sores HSV 2: Genital herpes

HSV Vaccine candidates • Subunit vaccine (phase II): efficacy in women • Live attenuated (current phase I) Challenges • Determining efficacy

Group B Streptococcus Perinatal transmission Asymptomatic colonization (21% pregnant women) Causes sepsis in newborns Generally treated with antibiotics during labor

GBS Vaccines • Pure polysaccharide • Protein conjugate vaccines (phases I, II) Challenges • Multiple serotypes





Link to the Jordan Report from NIP website

Vaccine-preventable diseases and adolescents: why so many cases?

Pertussis

Pertussis Impact Among Adolescents & Adults Pneumonia (2%) Rib fractures (1%) Hospitalization (~1%) Medical costs Missed school and work Impact on public health system Loss of sleep; loss of consciousness Weight loss

Why Adolescents & Adults need Pertussis Vaccine In 2003 pertussis vaccine levels in children 19 -35 months highest ever Pertussis cases continued to rise 2005 – 25, 616 pertussis cases, highest recorded since 1959 – 67% of cases - adolescent or adult – Overall incidence is 8. 7/100, 000 – Infants < 6 months 160. 8/100, 000 Pertussis immunity wanes in 5 -10 years

Pertussis Trends in the U. S. 1994 -2004 reported cases, 5 x higher 2001 -2005* - 109 pertussis-related deaths 88/109 or >80% who died were too young to have completed a primary series of DTa. P Source of infection most often an older child or adult in the household *2001 -15; 2002 -22; 2003 -18; 2004 -16; 2005 -38 CDC unpublished data

Why Pertussis in Adolescents? Waning immunity Improved diagnostics Improved surveillance

Tdap Vaccine Tdap vaccines licensed by FDA May and June 2005 ACIP Recommendations for adolescents in MMWR March 2006 ACIP Recommendations for adults in MMWR December 2006

Pertussis Vaccines - Tdap ® Boostrix (Glaxo. Smith. Kline) – Licensed May 3, 2005 – Single dose – Approved for persons 10 -18 years of age Adacel ® (sanofi pasteur) – Licensed June 10, 2005 – Single dose – Approved for persons 11 -64 years of age

Adolescent-Adult Pertussis Vaccination Objectives Primary – Protect vaccinated adolescents – Continue protection after completion of initial series Secondary – Reduce B. pertussis reservoir – Reduce pertussis incidence in other age groups

General Principles Use of Tdap and Td Tdap is preferred for protection against pertussis Tdap is licensed for ONE single dose at this time

Tdap Adolescent Recommendations Adolescents 11 -12 years of age should receive a single dose of Tdap instead of Td* Adolescents 13 -18 years with no Tdap should receive one dose as a catch-up booster instead of Td* *if the person has completed the recommended childhood DTa. P vaccination series, and has not yet received a Td booster

Persons >10 years with NO History of Primary Series Use 3 dose adult schedule but give Tdap for first dose of series Preferred schedule – #1 Tdap – #2 Td – at least 4 wks after dose #1 – #3 Td – at least 6 mos after dose #2

Tdap Recommended Uses Persons >10 yrs and adults who anticipate or have close contact with infants <12 months of age should receive one dose of Tdap can be used for tetanus prophylaxis wound management Use Tdap for next routine booster dose even if history of pertussis disease HCP with direct patient contact, esp. if <12 months age. Interval can be 2 years

Tdap Uses in Pregnancy* Safety data not available; registry is in progress If tetanus and diphtheria protection needed, give Td If pertussis risk present, give Tdap Pertussis risk in pregnancy = adolescents, pregnant HCP, child care providers of infants <12 months or vulnerable persons, living or working in area with increased pertussis Td and Tdap when vaccine should not be deferred to post partum, administration in 2 nd or 3 rd trimester is preferred *See section 3 -K in Adolescent Tdap ACIP Recommendations

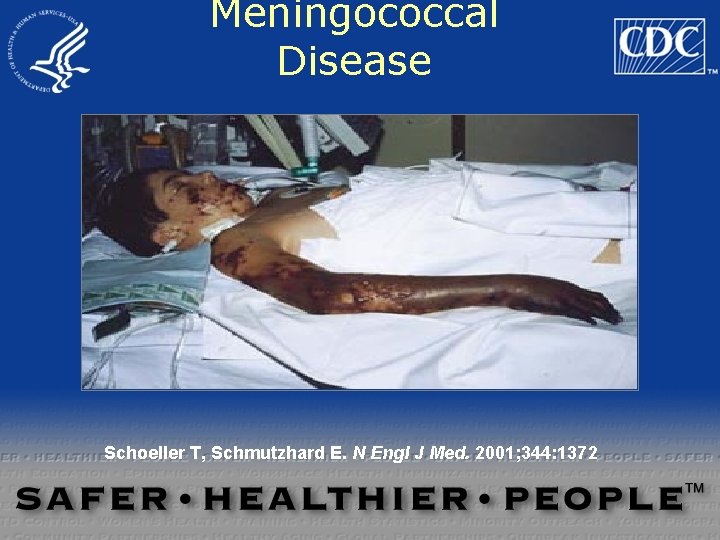
Meningococcal Disease Schoeller T, Schmutzhard E. N Engl J Med. 2001; 344: 1372

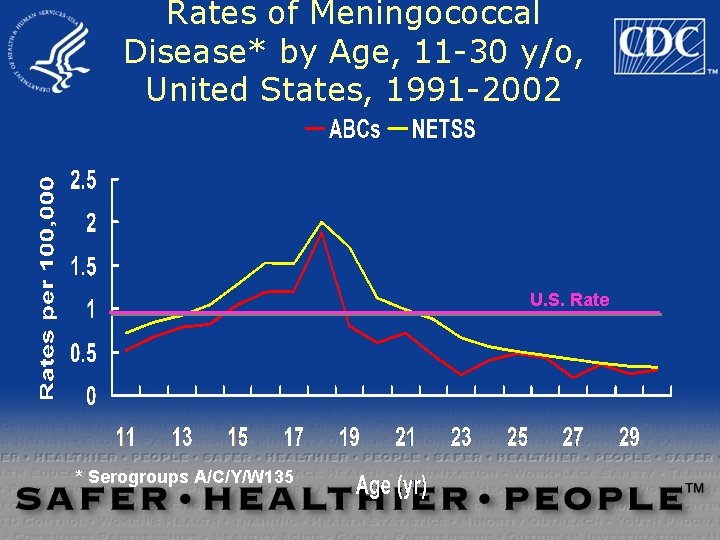
Rates of Meningococcal Disease* by Age, 11 -30 y/o, United States, 1991 -2002 U. S. Rate * Serogroups A/C/Y/W 135

Risk Factors for Meningococcal Disease in the United States Deficiencies in the terminal complement pathway Functional or anatomic asplenia HIV infection Smoking Passive exposure to smoke Upper respiratory tract infection Crowding


Meningococcal Disease Among Young Adults, United States, 1998 -1999 18 -23 years old 1. 4 / 100, 000 18 -23 years old not college student 100, 000 1. 4 / Freshmen 1. 92001; 286; 688/ 100, 000 Bruce et al, JAMA 93 Freshmen in dorm 5. 1 /

Approved by FDA January 2005

Meningococcal Conjugate Vaccine TM Menactra (sanofi pasteur) Quadrivalent (serogroups A, C, Y, W-135) conjugated to diphtheria toxoid Approved for persons 11 -55 years of age Schedule: 1 dose Administered by intramuscular injection

Meningococcal Conjugate Vaccine Approved only for persons 11 through 55 years of age Persons 2 -10 years of age >55 years at increased risk should receive the meningococcal POLYSACCHARIDE vaccine Meningococcal vaccine is not routinely recommended for persons 2 -10 years of age or older than 55 years who are not in a high risk group

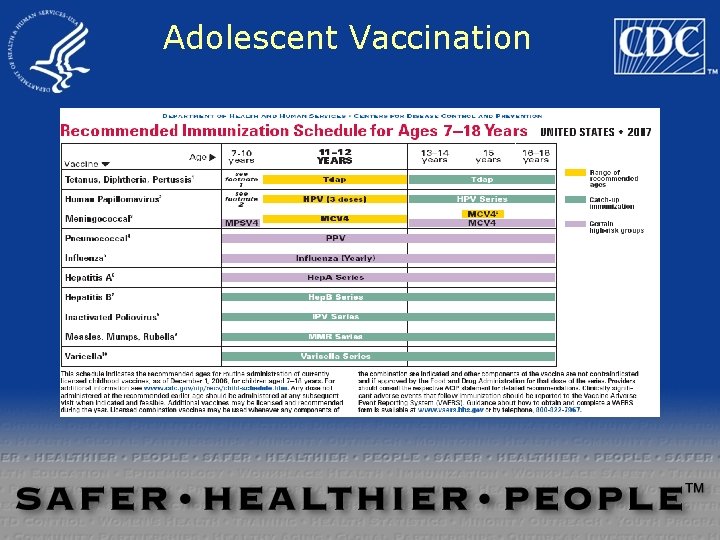
Meningococcal Vaccine Recommendations Recommended for: – all persons at the preadolescent visit (ages 1112 years) – persons about to enter high school (age 15 years) – college freshmen living in a dormitory – other adolescents who wish to reduce their risk for meningococcal disease MMWR 2005; 54(RR-7)

Meningococcal Vaccine Recommendations Recommended for certain high -risk persons: – military recruits – certain research and laboratory personnel – travelers to and U. S. citizens residing in countries in which N. meningitidis is hyperendemic or epidemicterminal complement component deficiency – functional or anatomic asplenia – HIV infection (“should be considered”) MMWR 2005; 54(RR-7); 1 -21


Meningococcal Conjugate Vaccine (MCV) and GBS MCV approved by FDA in January 2005 15 cases of GBS among 11 -19 year olds within 6 weeks of MCV FDA/CDC advisory issued September 30, 2005 No change in vaccine recommendations as of October 20, 2005* *except to avoid vaccination of persons with a history of GBS who are not at increased risk of infection

Newsweek: 1 May 2006 Dubuque, Iowa 1956 Philadelphia

Why Mumps in Adolescents? Highly communicable Imperfect vaccine efficacy

Mumps Vaccine Efficacy • 70 -80% after one dose • 90 -95% after two doses Therefore: • 50 -100 of 1, 000 immunized persons will be infected • Of 100 people, 98 are immunized § 5 or 5% of the 98 get mumps § 2 unvaccinated get mumps § 5 of the 7 total who get mumps will be immunized

Mumps Prevention Immunization – TWO doses for school age children – Ensure adult immunity – Healthcare workers need immunity!! • HCWs need TWO doses MMR or proof of immunity Identify and isolate ill persons for ~ 9 days – note location, date for epi-link Identify and vaccinate susceptible contacts Practice good hygiene

Adolescent Vaccination


Questions?
 Andrew kroger
Andrew kroger Andrew kroger
Andrew kroger New frontiers beliefs
New frontiers beliefs Unix internals: the new frontiers
Unix internals: the new frontiers Boeing research and technology
Boeing research and technology Kroger organizational structure
Kroger organizational structure Cheminstry
Cheminstry Durant motors stock 1920s
Durant motors stock 1920s Kroger vink notation examples
Kroger vink notation examples Kroger foods stock 1920
Kroger foods stock 1920 Adolescents defenition
Adolescents defenition Infant child and adolescent berk 8th edition chapter 1
Infant child and adolescent berk 8th edition chapter 1 Lara berk
Lara berk Poultry vaccination schedule
Poultry vaccination schedule Vaccination schedule in palestine
Vaccination schedule in palestine Dog vaccination perry county
Dog vaccination perry county 10 rights of medication administration
10 rights of medication administration Vaccination bruxelles
Vaccination bruxelles Mandatory vaccination
Mandatory vaccination Vaccination xard
Vaccination xard Difference between spermatogenesis and oogenesis
Difference between spermatogenesis and oogenesis Meaning of variolation
Meaning of variolation Pcv vaccine route
Pcv vaccine route Epi schedule bangladesh
Epi schedule bangladesh Directions for producing mhcs come from
Directions for producing mhcs come from Sleeping princesses
Sleeping princesses Mark anthony fernandez vaccination
Mark anthony fernandez vaccination Lokstallarna jönköping vaccination
Lokstallarna jönköping vaccination Chapter 9 frontiers of biotechnology
Chapter 9 frontiers of biotechnology Richard walker frontiers
Richard walker frontiers Frontiers of biotechnology chapter 9
Frontiers of biotechnology chapter 9 Frontiers of biotechnology chapter 9
Frontiers of biotechnology chapter 9 Frontiers in chemical engineering
Frontiers in chemical engineering Hochedlinger
Hochedlinger Frontiers in bioscience impact factor
Frontiers in bioscience impact factor Weakening frontiers meaning
Weakening frontiers meaning Frontiers of information technology
Frontiers of information technology Frontiers in bioinformatics
Frontiers in bioinformatics Frontiers
Frontiers Hfsp letter of intent
Hfsp letter of intent Why did andrew jackson oppose the national bank
Why did andrew jackson oppose the national bank National unification and the national state
National unification and the national state New zealand national sport
New zealand national sport A new national identity section 1
A new national identity section 1 A new national identity section 1
A new national identity section 1 Rules for speech punctuation
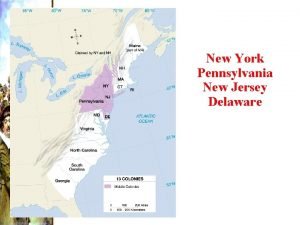
Rules for speech punctuation New york, new jersey, pennsylvania, and delaware
New york, new jersey, pennsylvania, and delaware Fresh oil, new wine scripture
Fresh oil, new wine scripture Marquee new hartford ny
Marquee new hartford ny Both new hampshire and new york desire more territory
Both new hampshire and new york desire more territory New-old approach to creating new ventures
New-old approach to creating new ventures Kotler keller marketing management
Kotler keller marketing management