Vaccination Errors Oops Reported to the Vaccine Adverse















- Slides: 27

Vaccination Errors (Oops!) Reported to the Vaccine Adverse Event Reporting System (VAERS), 2000 -2011 Beth Hibbs, RN, MPH Immunization Safety Office - Division Of Healthcare Quality Promotion, Centers for Disease Control and Prevention National Immunization Conference 2012 The findings in this presentation are those of the authors and do not necessarily represent the official position of CDC or FDA 1

Overview q q q Background Objective Methods Findings Summary Conclusions 2

Background Vaccination Errors q Institute of Medicine (IOM) reports focused national attention on medical errorsa q MMWR –in past ten years, 3 notices on vaccination errorsb q Increasing complexity of the immunization schedule a Preventing Medication Errors IOM 2007, http: //www. nap. edu/ b Notice to Readers: Inadvertent Intradermal Administration of Tetanus Toxoid- -Containing Vaccines Instead of Tuberculosis Skin Tests MMWR 2004; * Inadvertent Misadministration of Meningococcal Conjugate Vaccine MMWR Sept 2006; *Preventing Tetanus, Diphtheria, and Pertussis Among Adolescents: Use of Tetanus Toxoid, Reduced Diphtheria Toxoid and acellular Pertussis Vaccines. www. cdc. gov/mmwr/PDF/rr/rr 5503. pdf (Page 27)* 3

Definitions q Vaccination Error § Any preventable event that may cause or lead to inappropriate use or patient harm. Such events may be related to professional practice, immunization products, (vials, needle, syringes), storage, dispensing and administration* q Vaccine Adverse Health Event § Health effects that occur after immunization that may or may not be causally related to the vaccine *CDC Immunization Safety Office, VAERS Medication Error Study workgroup. Adapted in part from U. S. Pharmacopeia (USP) medical error definition from http: //www. usp. org/sites/default/files/usp_pdf/EN/members/patient. Safety. pdf 4

National Organizations that Accept Reports of Vaccination Errors q Vaccine Adverse Event Reporting System (VAERS) § Accepts reports about adverse events following immunization. 800 -822 -7967 www. vaers. hhs. gov q Med. Watch § Accepts reports about products regulated by the FDA, including drugs and medical devices. 800 -332 -1088 www. fda. gov/medwatch/how. htm q Institute for Safe Medication Practices (ISMP) § Medication Error Reporting Program (MERP) accepts reports related to med errors. 215 -947 -7797 www. ismp. org Some mistakes all the world can see…. . Other errors need to be reported to better understand prevent them 5

Vaccine Adverse Event Reporting System (VAERS) q Authorized by National Childhood Vaccine Injury Act of 1986 § Jointly administered CDC and FDA q National, post-marketing, passive reporting system for adverse events occurring after receipt of USlicensed vaccines § Receives average ~33, 000* reports/year (2007 -2011) *Numbers include both US and foreign reports, primary and non-primary 6

VAERS Studies of Vaccination Errors q 1994 -2001 an analysis of vaccination error reportsa § 49 total reports of vaccination errors in VAERS § Most frequent error were wrong vaccine given § 42/49 adverse health events reported, most frequent was injection site reaction(12), fever (3) and irritability(3). Number of serious reports not reported. q 2001 -2002 follow up study of the 1994 -2001 reportb § 119 errors were reported to VAERS (26 actual or true errors) § 76 were not actual errors (37 data entry errors, 30 reporter errors, 9 “off-label” use by prescribing physician) § The most frequent error was wrong vaccine given § A wide variety of adverse health events were reported (i. e. cellulitis, seizure, wheezing, ). Number of serious reports not reported. a Varricchio F. Medication errors reported to the vaccine adverse event reporting system (VAERS). Vaccine. 2002 Aug 19; 20(25 -26): 3049 -51. b Varricchio F, Reed J; Follow-up study of medication errors reported to the vaccine adverse event reporting system (VAERS). South Med J. 2006 May; 99(5): 486 -9. 7

Objective q To describe the types and frequency of vaccination errors received by VAERS in the U. S. 2000 -2011 q Description of selected findings § 2006 -2011 Rotavirus vaccine § 2011 -2012 trivalent influenza vaccine (TIV) 8

Methods q Search inclusion criteria: § All VAERS U. S. primary reports with a vaccination error code § Report receipt dates: 01/01/2000 -12/31/2011 § Coded vaccination error and health events for VAERS reports using Medical Dictionary for Regulatory Activities (Med. DRA) • 39 Med. DRA codes describing vaccination errors q Classified error reports into 11 error groups q Reviewed and characterized selected reports 9

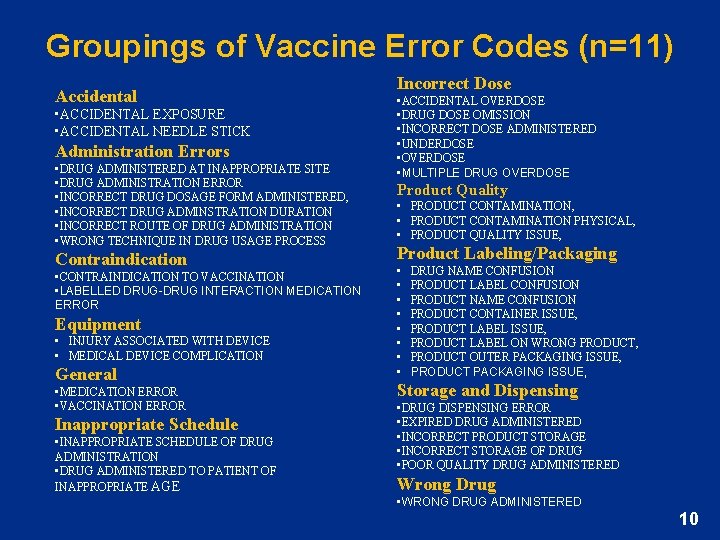
Groupings of Vaccine Error Codes (n=11) Accidental • ACCIDENTAL EXPOSURE • ACCIDENTAL NEEDLE STICK Administration Errors • DRUG ADMINISTERED AT INAPPROPRIATE SITE • DRUG ADMINISTRATION ERROR • INCORRECT DRUG DOSAGE FORM ADMINISTERED, • INCORRECT DRUG ADMINSTRATION DURATION • INCORRECT ROUTE OF DRUG ADMINISTRATION • WRONG TECHNIQUE IN DRUG USAGE PROCESS Contraindication • CONTRAINDICATION TO VACCINATION • LABELLED DRUG-DRUG INTERACTION MEDICATION ERROR Equipment • INJURY ASSOCIATED WITH DEVICE • MEDICAL DEVICE COMPLICATION General • MEDICATION ERROR • VACCINATION ERROR Inappropriate Schedule • INAPPROPRIATE SCHEDULE OF DRUG ADMINISTRATION • DRUG ADMINISTERED TO PATIENT OF INAPPROPRIATE AGE Incorrect Dose • ACCIDENTAL OVERDOSE • DRUG DOSE OMISSION • INCORRECT DOSE ADMINISTERED • UNDERDOSE • OVERDOSE • MULTIPLE DRUG OVERDOSE Product Quality • PRODUCT CONTAMINATION, • PRODUCT CONTAMINATION PHYSICAL, • PRODUCT QUALITY ISSUE, Product Labeling/Packaging • • DRUG NAME CONFUSION PRODUCT LABEL CONFUSION PRODUCT NAME CONFUSION PRODUCT CONTAINER ISSUE, PRODUCT LABEL ON WRONG PRODUCT, PRODUCT OUTER PACKAGING ISSUE, PRODUCT PACKAGING ISSUE, Storage and Dispensing • DRUG DISPENSING ERROR • EXPIRED DRUG ADMINISTERED • INCORRECT PRODUCT STORAGE • INCORRECT STORAGE OF DRUG • POOR QUALITY DRUG ADMINISTERED Wrong Drug • WRONG DRUG ADMINISTERED 10

VAERS Surveillance Definitions for “Serious” versus “Non-serious” Reports A check in box 8 of the VAERS form for these categories = “Serious" 11

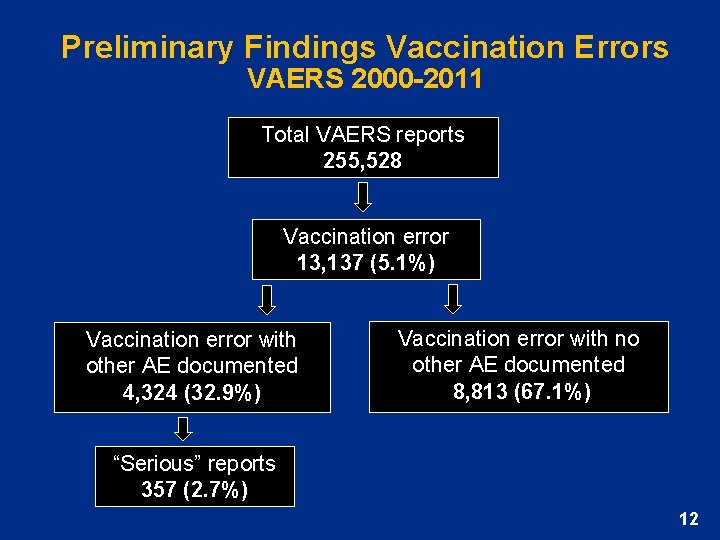
Preliminary Findings Vaccination Errors VAERS 2000 -2011 Total VAERS reports 255, 528 Vaccination error 13, 137 (5. 1%) Vaccination error with other AE documented 4, 324 (32. 9%) Vaccination error with no other AE documented 8, 813 (67. 1%) “Serious” reports 357 (2. 7%) 12

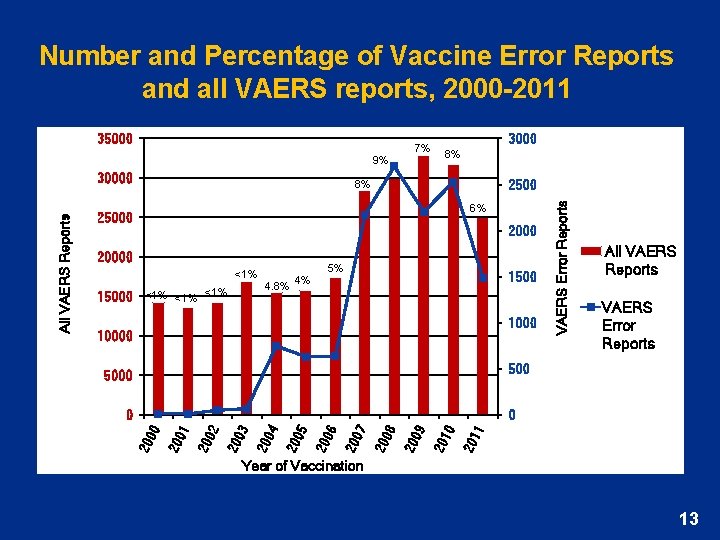
Number and Percentage of Vaccine Error Reports and all VAERS reports, 2000 -2011 35000 9% 3000 8% 6% 25000 20000 <1% 15000 <1% <1% 4. 8% 4% 5% 1500 10000 VAERS Error Reports 2500 8% All VAERS Reports VAERS Error Reports 5000 11 20 10 20 09 20 08 20 07 20 06 20 05 20 04 20 03 20 02 20 20 01 0 00 0 20 All VAERS Reports 30000 7% Year of Vaccination 13

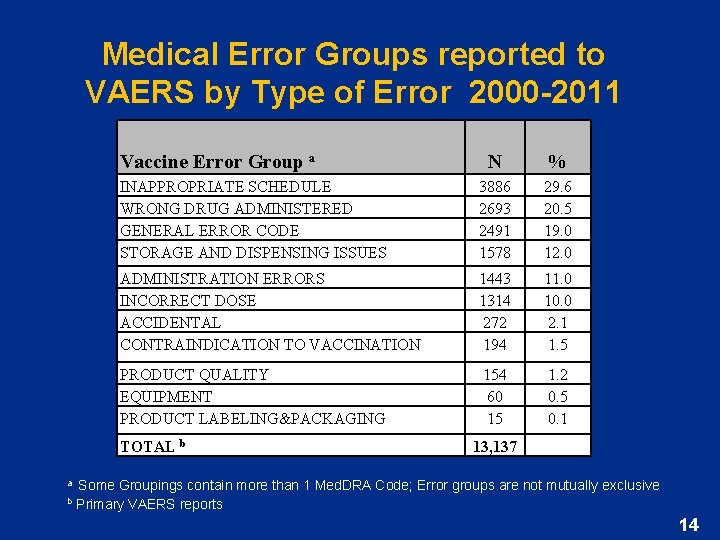
Medical Error Groups reported to VAERS by Type of Error 2000 -2011 Vaccine Error Group a N % INAPPROPRIATE SCHEDULE WRONG DRUG ADMINISTERED GENERAL ERROR CODE STORAGE AND DISPENSING ISSUES 3886 2693 2491 1578 29. 6 20. 5 19. 0 12. 0 ADMINISTRATION ERRORS INCORRECT DOSE ACCIDENTAL CONTRAINDICATION TO VACCINATION 1443 1314 272 194 11. 0 10. 0 2. 1 1. 5 PRODUCT QUALITY EQUIPMENT PRODUCT LABELING&PACKAGING 154 60 15 1. 2 0. 5 0. 1 TOTAL b 13, 137 Some Groupings contain more than 1 Med. DRA Code; Error groups are not mutually exclusive a b Primary VAERS reports 14

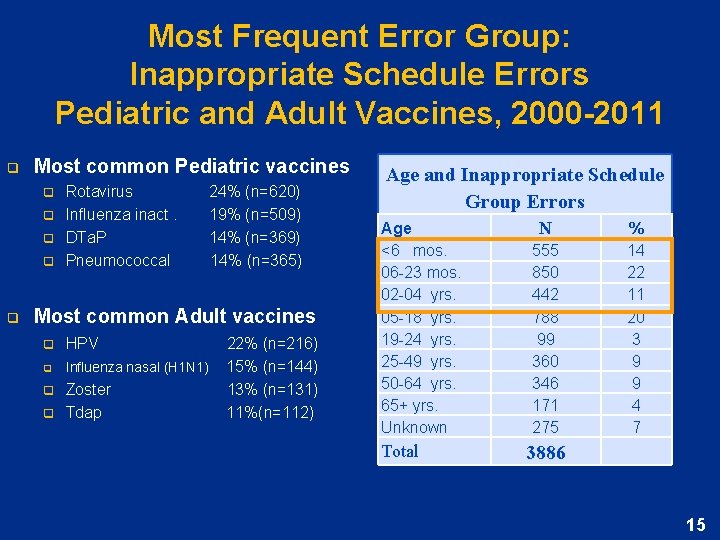
Most Frequent Error Group: Inappropriate Schedule Errors Pediatric and Adult Vaccines, 2000 -2011 q Most common Pediatric vaccines q q q Rotavirus 24% (n=620) Influenza inact. 19% (n=509) DTa. P 14% (n=369) Pneumococcal 14% (n=365) Most common Adult vaccines q q HPV 22% (n=216) Influenza nasal (H 1 N 1) 15% (n=144) Zoster 13% (n=131) Tdap 11%(n=112) Age and Inappropriate Schedule Group Errors Age <6 mos. 06 -23 mos. 02 -04 yrs. 05 -18 yrs. 19 -24 yrs. 25 -49 yrs. 50 -64 yrs. 65+ yrs. Unknown Total N 555 850 442 788 99 360 346 171 275 % 14 22 11 20 3 9 9 4 7 3886 15

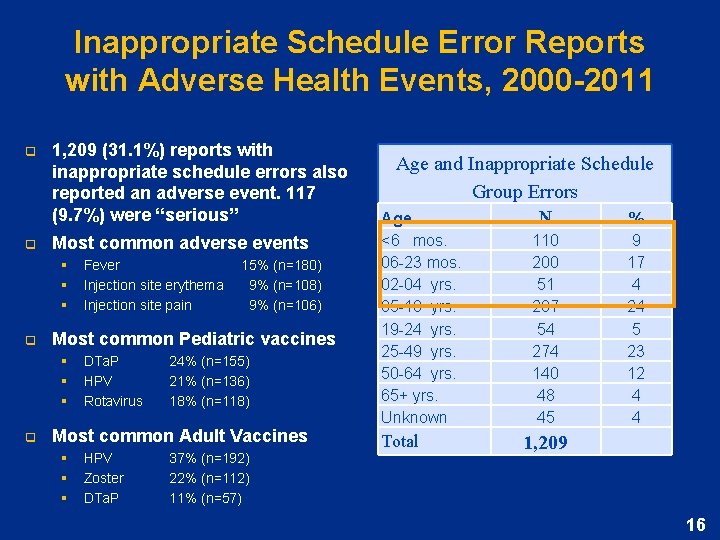
Inappropriate Schedule Error Reports with Adverse Health Events, 2000 -2011 q q 1, 209 (31. 1%) reports with inappropriate schedule errors also reported an adverse event. 117 (9. 7%) were “serious” Most common adverse events § § § q 15% (n=180) 9% (n=108) 9% (n=106) Most common Pediatric vaccines § § § q Fever Injection site erythema Injection site pain DTa. P HPV Rotavirus 24% (n=155) 21% (n=136) 18% (n=118) Most common Adult Vaccines § § § HPV Zoster DTa. P 37% (n=192) 22% (n=112) 11% (n=57) Age and Inappropriate Schedule Group Errors Age <6 mos. 06 -23 mos. 02 -04 yrs. 05 -18 yrs. 19 -24 yrs. 25 -49 yrs. 50 -64 yrs. 65+ yrs. Unknown Total N 110 200 51 287 54 274 140 48 45 % 9 17 4 24 5 23 12 4 4 1, 209 16

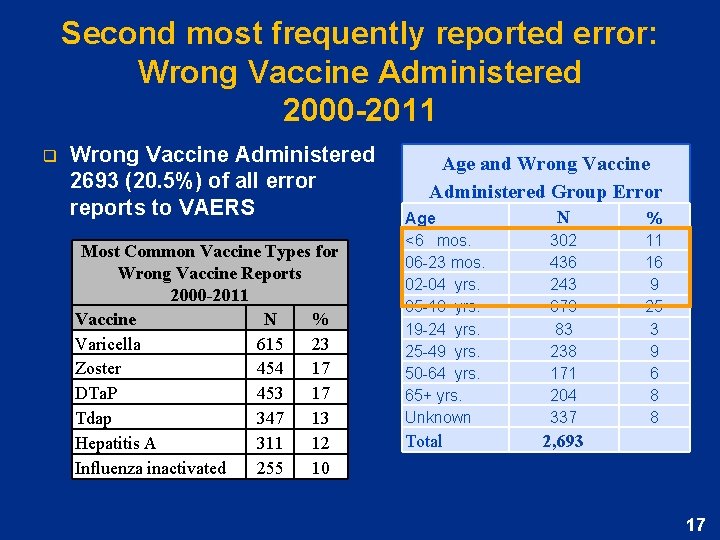
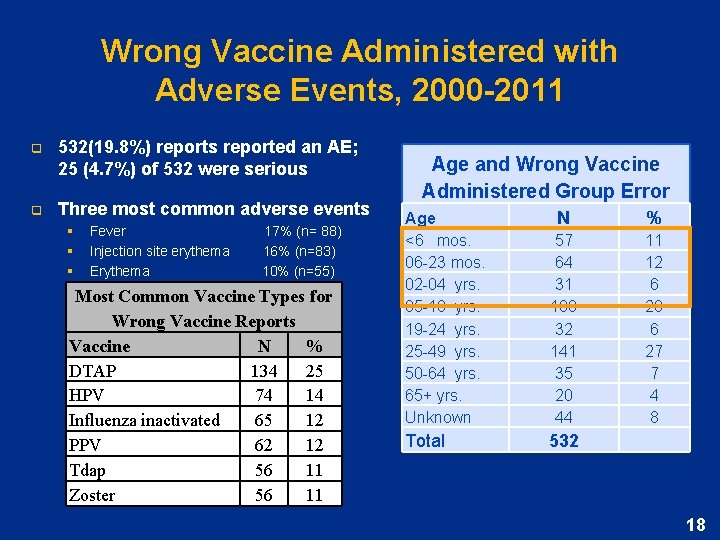
Second most frequently reported error: Wrong Vaccine Administered 2000 -2011 q Wrong Vaccine Administered 2693 (20. 5%) of all error reports to VAERS Most Common Vaccine Types for Wrong Vaccine Reports 2000 -2011 Vaccine N % Varicella 615 23 Zoster 454 17 DTa. P 453 17 Tdap 347 13 Hepatitis A 311 12 Influenza inactivated 255 10 Age and Wrong Vaccine Administered Group Error Age <6 mos. 06 -23 mos. 02 -04 yrs. 05 -18 yrs. 19 -24 yrs. 25 -49 yrs. 50 -64 yrs. 65+ yrs. Unknown Total N 302 436 243 679 83 238 171 204 337 % 11 16 9 25 3 9 6 8 8 2, 693 17

Wrong Vaccine Administered with Adverse Events, 2000 -2011 q 532(19. 8%) reports reported an AE; 25 (4. 7%) of 532 were serious q Three most common adverse events § § § Fever 17% (n= 88) Injection site erythema 16% (n=83) Erythema 10% (n=55) Most Common Vaccine Types for Wrong Vaccine Reports Vaccine N % DTAP 134 25 HPV 74 14 Influenza inactivated 65 12 PPV 62 12 Tdap 56 11 Zoster 56 11 Age and Wrong Vaccine Administered Group Error Age <6 mos. 06 -23 mos. 02 -04 yrs. 05 -18 yrs. 19 -24 yrs. 25 -49 yrs. 50 -64 yrs. 65+ yrs. Unknown N % 57 64 31 108 32 141 35 20 44 11 12 6 20 6 27 7 4 8 Total 532 18

Wrong Vaccine Administered “Mix-ups” for 100 Randomly Selected Reports q The most frequent vaccine combinations “mix-ups” § Varicella – Zostavax (n=25) § DTa. P – Tdap (n=11) § Pneumovax – Prevnar (n=7) q 10 reported an adverse health event: § Two serious reports (meningitis; otitis media/influenza) § Non-serious reports • • • Injection site reaction (3) Fever (2) Respiratory (1) Constitutional symptoms (1) Allergic (1) 19

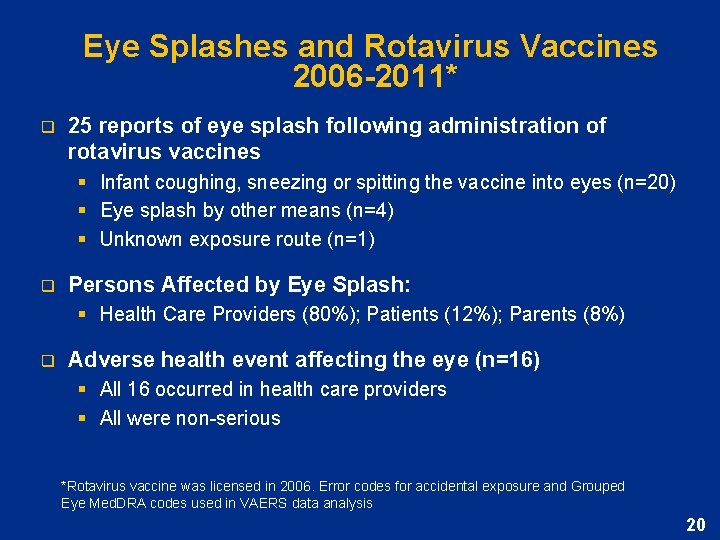
Eye Splashes and Rotavirus Vaccines 2006 -2011* q 25 reports of eye splash following administration of rotavirus vaccines § Infant coughing, sneezing or spitting the vaccine into eyes (n=20) § Eye splash by other means (n=4) § Unknown exposure route (n=1) q Persons Affected by Eye Splash: § Health Care Providers (80%); Patients (12%); Parents (8%) q Adverse health event affecting the eye (n=16) § All 16 occurred in health care providers § All were non-serious *Rotavirus vaccine was licensed in 2006. Error codes for accidental exposure and Grouped Eye Med. DRA codes used in VAERS data analysis 20

Seasonal Trivalent Inactivated Influenza Vaccine (TIV) and Vaccine Administered at Inappropriate Site Errors, 2011 -2012 q 89 reports of Vaccine Administration Group Errors § 50 (56%) were Vaccine Administered at Inappropriate Site • 49 (98%) reported an Adverse Health Event o Pain in extremity (43%); Injection site pain (37%); Pain (28%); Musculoskeletal pain (18 %); Injected limb mobility decreased (16%); Bursitis (10%) o Serious reports (n=2) • • (VAERS #443753)“Intense pain 8 hours after vaccination… Vaccine was administered high on the left shoulder. ” The pain persisted for several weeks “MRI showed a split rotor cup” surgery required (VAERS #447139)“shot given high in shoulder, extreme pain while receiving , Could not move or use arm immediately after shot” – ”surgery required for extreme inflammation” 21

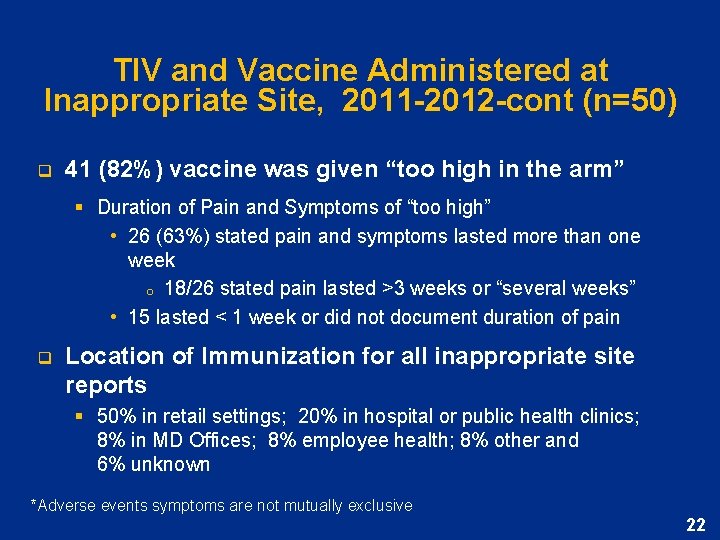
TIV and Vaccine Administered at Inappropriate Site, 2011 -2012 -cont (n=50) q 41 (82%) vaccine was given “too high in the arm” § Duration of Pain and Symptoms of “too high” • 26 (63%) stated pain and symptoms lasted more than one week o 18/26 stated pain lasted >3 weeks or “several weeks” • 15 lasted < 1 week or did not document duration of pain q Location of Immunization for all inappropriate site reports § 50% in retail settings; 20% in hospital or public health clinics; 8% in MD Offices; 8% employee health; 8% other and 6% unknown *Adverse events symptoms are not mutually exclusive 22

Summary q Vaccination Error reports have increased in VAERS during the past 11 years from 0. 07% to 9% q “Inappropriate Schedule of Vaccine Administration” § Most commonly reported error, especially among children q Two-thirds of vaccination error reports have no reported adverse health event § However, errors can affect cost, convenience, effectiveness, and confidence in vaccination programs q Potential areas for public health prevention/intervention § Wrong vaccine “mix-ups” more common between similar vaccines that are given to children and adults (Varicella-Zostavax, DTa. P-Tdap) § Eye splashes and rotavirus vaccine § Inappropriate site—”too high in arm” and Influenza vaccines TIV 23

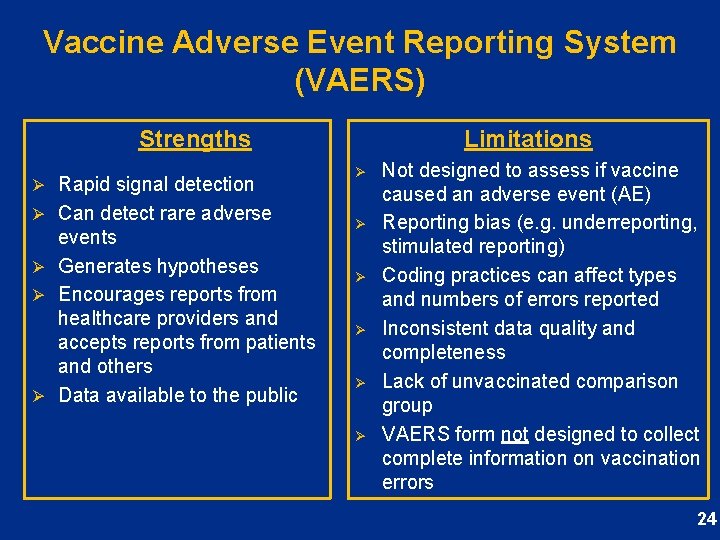
Vaccine Adverse Event Reporting System (VAERS) Strengths Ø Rapid signal detection Ø Can detect rare adverse events Ø Generates hypotheses Ø Encourages reports from healthcare providers and accepts reports from patients and others Ø Data available to the public Limitations Ø Ø Ø Not designed to assess if vaccine caused an adverse event (AE) Reporting bias (e. g. underreporting, stimulated reporting) Coding practices can affect types and numbers of errors reported Inconsistent data quality and completeness Lack of unvaccinated comparison group VAERS form not designed to collect complete information on vaccination errors 24

Conclusions q Vaccination Errors are an important area in vaccine safety research and surveillance. Continued study will be key in understanding and developing strategies to prevent them. q Focus of continued study in VAERS: § Errors that may be associated with an adverse health outcome § Frequency of the vaccination error § Preventability of the vaccination error 25

Resources for Preventing Vaccination Errors q Proper Storage and Handling of Vaccines § Vaccine Storage and Handling Guide (tool kit under revision), Pink Book Chapter 5, www. cdc. gov § http: //www. cdc. gov/vaccines/recs/storage/default. htm q Identify Contraindications & Precautions § Immunization Screening Questionnaires, www. cdc. gov q Proper Vaccine Administration–Right patient, Right time, Right dose, Right route, Right drug* § General Recommendations on Immunization www. cdc. gov § http: //www. cdc. gov/vaccines/recs/vac-admin/default. htm q Report Clinically Significant Vaccination Errors to VAERS § VAERS www. vaers. hhs. gov *The five rights of medication administration Nursing literature

Acknowledgements q q q q Pedro Moro Paige Lewis Oidda Museru Elaine Miller Talia Yue Theresa Harrington Claudia Vellozzi Karen Broder 27