Utilising multiplecompound study designs to achieve a higher










- Slides: 18

Utilising multiple-compound study designs to achieve a higher investment on tried and tested methods Joanna Segieth Ph. D, MTOPRA

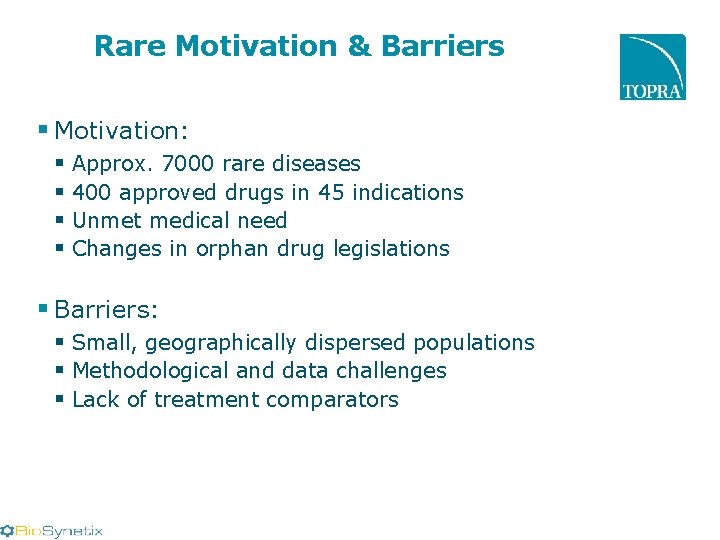
Rare Motivation & Barriers § Motivation: § Approx. 7000 rare diseases § 400 approved drugs in 45 indications § Unmet medical need § Changes in orphan drug legislations § Barriers: § Small, geographically dispersed populations § Methodological and data challenges § Lack of treatment comparators

Terminology

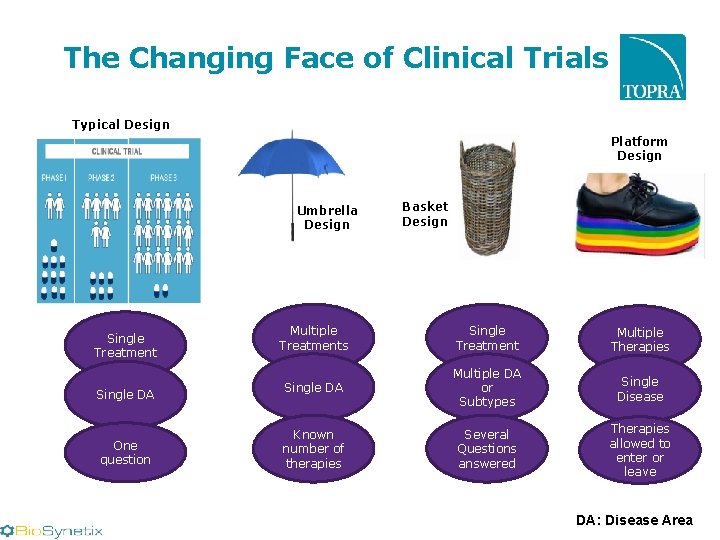
The Changing Face of Clinical Trials Typical Design Platform Design Umbrella Design Single Treatment Single DA One question Basket Design Multiple Treatments Single Treatment Multiple Therapies Single DA Multiple DA or Subtypes Single Disease Known number of therapies Several Questions answered Therapies allowed to enter or leave DA: Disease Area

Case Study 1: LOXO-101

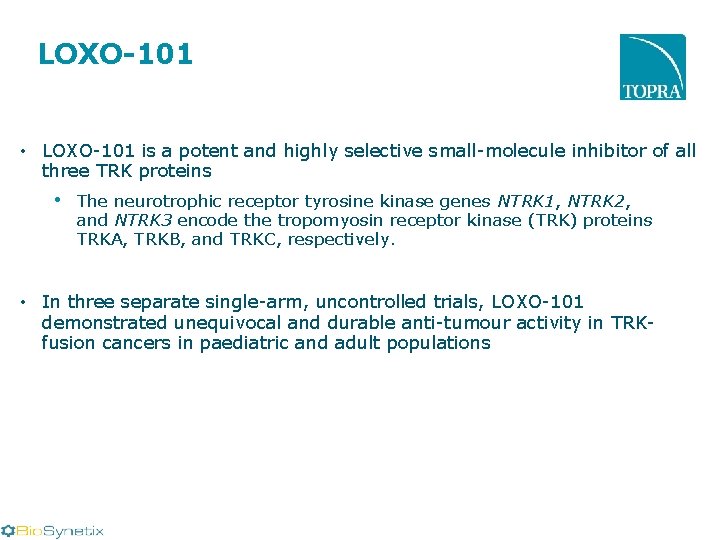
LOXO-101 • LOXO-101 is a potent and highly selective small-molecule inhibitor of all three TRK proteins • The neurotrophic receptor tyrosine kinase genes NTRK 1, NTRK 2, and NTRK 3 encode the tropomyosin receptor kinase (TRK) proteins TRKA, TRKB, and TRKC, respectively. • In three separate single-arm, uncontrolled trials, LOXO-101 demonstrated unequivocal and durable anti-tumour activity in TRKfusion cancers in paediatric and adult populations

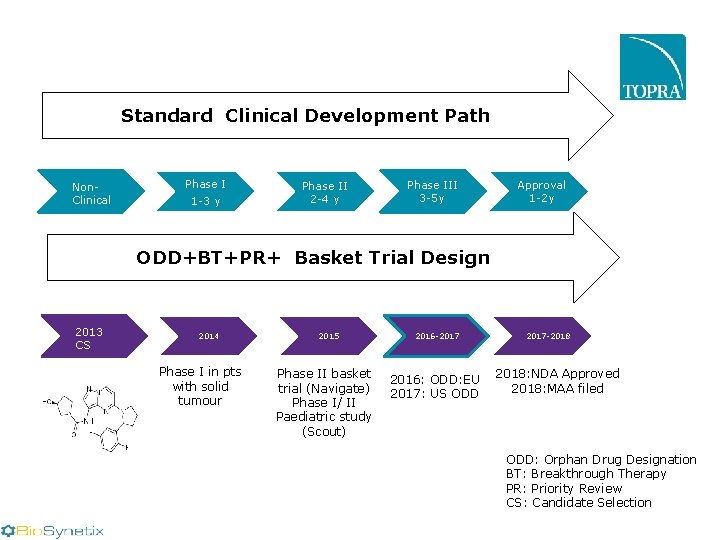
Standard Clinical Development Path Non. Clinical Phase I 1 -3 y Phase II 2 -4 y Confirmatory Phase III phase III 3 -5 y (2 - 5 y) Approval 1 -2 y ODD+BT+PR+ Basket Trial Design 2013 CS 2014 Phase I in pts with solid tumour 2015 Phase II basket trial (Navigate) Phase I/ II Paediatric study (Scout) 2016 -2017 2016: ODD: EU 2017: US ODD 2017 -2018: NDA Approved 2018: MAA filed ODD: Orphan Drug Designation BT: Breakthrough Therapy PR: Priority Review CS: Candidate Selection

Tumour -Agnostic Label FDA Approved: Yes (First approved November 26, 2018) Brand name: Vitrakvi Generic name: larotrectinib Dosage form: Capsules and Oral Solution Company: Loxo Oncology, Inc. Treatment for: TRK Fusion Cancers Vitrakvi (larotrectinib) is an oral selective tropomyosin receptor kinase (TRK) inhibitor for the treatment solid tumours harbouring NTRK-fusion proteins. Tumour –type –agnostic drug development is not a universal model and may only be applicable to drugs with high clinical response rates and rare tumour types

Case Study 2: Rare Basket

Rare protocol/basket trial design for a first-in-human trial • TNT 009 is a first-in-class monoclonal antibody that selectively inhibits the Classical Complement pathway by targeting C 1 s, a serine protease within the C 1 -complex in the Complement pathway of the immune system. • TNT 009 is currently in clinical development for the treatment of Cold Agglutinin Disease (CAD), a rare disease in hematology. Other diseases are known to share the basic underlying mechanism addressed by TNT 009 - the potential for utilising basket trial methodology exists • First-in-human-trial which facilitates the transition from phase 1 a in healthy volunteers to phase I b in patients with rare complement –mediated disorders utilising the concept of basket trials North Therapeutics

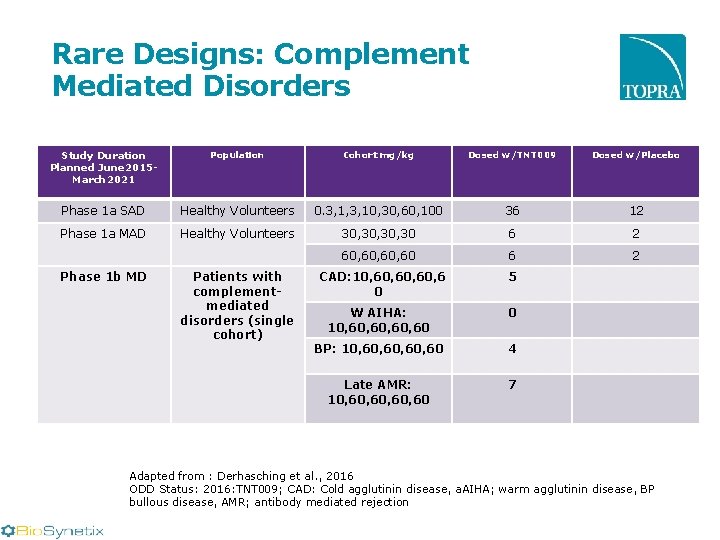
Rare Designs: Complement Mediated Disorders Study Duration Planned June 2015 March 2021 Population Cohort mg/kg Dosed w/TNT 009 Dosed w/Placebo Phase 1 a SAD Healthy Volunteers 0. 3, 1, 3, 10, 30, 60, 100 36 12 Phase 1 a MAD Healthy Volunteers 30, 30, 30 6 2 60, 60, 60 6 2 Phase 1 b MD Patients with complementmediated disorders (single cohort) CAD: 10, 60, 6 0 5 W AIHA: 10, 60, 60 0 BP: 10, 60, 60 4 Late AMR: 10, 60, 60 7 Adapted from : Derhasching et al. , 2016 ODD Status: 2016: TNT 009; CAD: Cold agglutinin disease, a. AIHA; warm agglutinin disease, BP bullous disease, AMR; antibody mediated rejection

Rare Basket Trials • Basket study design is suitable to rare diseases sharing a common genetic aberration • and/or • Rare diseases sharing a similar pathophysiological mechanism targeted by a monoclonal antibody against a single target • Pragmatic approach to prioritization of target indications to pursue for larger clinical development programmes i. e. ‘play the winner’

What about multi-compound study designs such as platform studies?

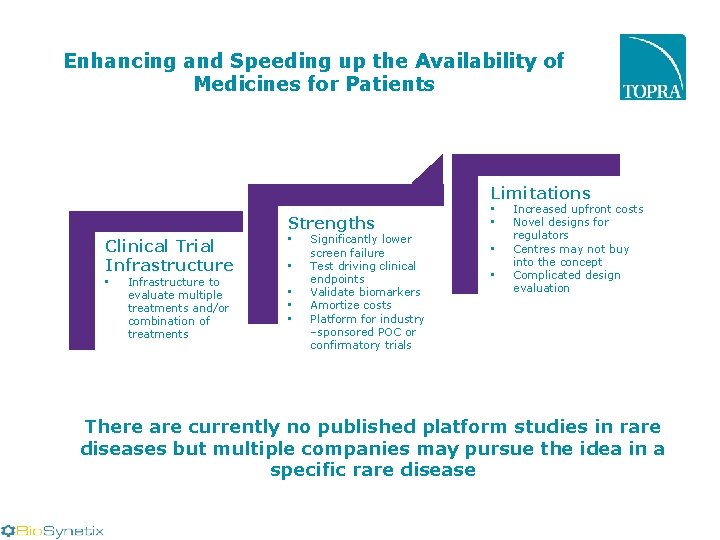
Enhancing and Speeding up the Availability of Medicines for Patients Limitations Strengths Clinical Trial Infrastructure • Infrastructure to evaluate multiple treatments and/or combination of treatments • • • Significantly lower screen failure Test driving clinical endpoints Validate biomarkers Amortize costs Platform for industry –sponsored POC or confirmatory trials • • Increased upfront costs Novel designs for regulators Centres may not buy into the concept Complicated design evaluation There are currently no published platform studies in rare diseases but multiple companies may pursue the idea in a specific rare disease

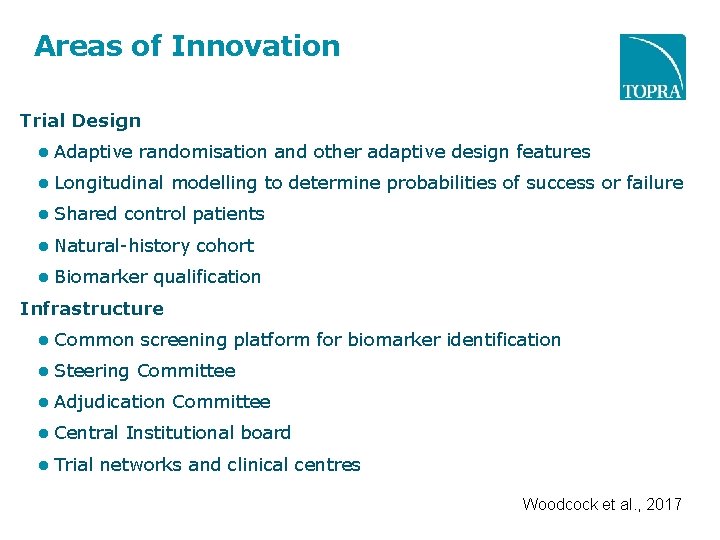
Areas of Innovation Trial Design ● Adaptive randomisation and other adaptive design features ● Longitudinal modelling to determine probabilities of success or failure ● Shared control patients ● Natural-history cohort ● Biomarker qualification Infrastructure ● Common screening platform for biomarker identification ● Steering Committee ● Adjudication Committee ● Central Institutional board ● Trial networks and clinical centres Woodcock et al. , 2017

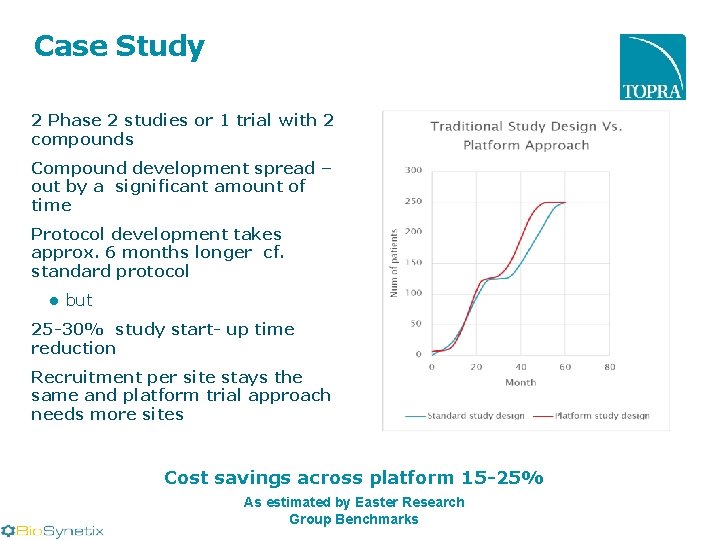
Case Study 2 Phase 2 studies or 1 trial with 2 compounds Compound development spread – out by a significant amount of time Protocol development takes approx. 6 months longer cf. standard protocol ● but 25 -30% study start- up time reduction Recruitment per site stays the same and platform trial approach needs more sites Cost savings across platform 15 -25% As estimated by Easter Research Group Benchmarks

Conclusion Why might the innovative study designs be of interest in rare diseases? • Rapid comparison of existing therapies • Reduces clinical testing cycle in particular in a phase II setting • Reduces the required number of patients for testing • Rapid delivery of effective therapies to patients

Helping Patients to Achieve Better Outcomes ! Connect Collaborate Thank you ! Cure joanna. segieth@biosynetix. com