USP 797 and 800 Update PATRICIA C KIENLE


































- Slides: 49

USP <797> and <800> Update PATRICIA C. KIENLE, RPH, MPA, FASHP

Disclosure Patricia Kienle is a member of the USP Compounding Expert Committee but this talk is not affiliated with nor endorsed by USP

Objectives - Pharmacists Cite the status of the USP compounding chapters Explain the types of facilities required for sterile compounding and for hazardous drug compounding Define the types of environmental monitoring required for sterile and hazardous compounding State the document that must be used to develop your hazardous drug list List three alternative containment strategies and/or work practices that can be used to mitigate risks of hazardous drugs that are not antineoplastics

Objectives - Technicians Differentiate the USP compounding chapters that are official now, and those that will be official wthin the next year State the two types of IV rooms where compounded sterile preparations can be mixed List the two required environmental monitoring tests required by USP <797> State the organization that maintains the list of drugs that are hazardous to healthcare personnel State three types of engineering controls required by USP <800>

Is your pharmacy compliant with USP <797> and <800>? A. Yes, compliant with both B. Compliant with <797> and working on <800> C. Partially compliant with <797> and working on <800> D. We’ve got a ways to go

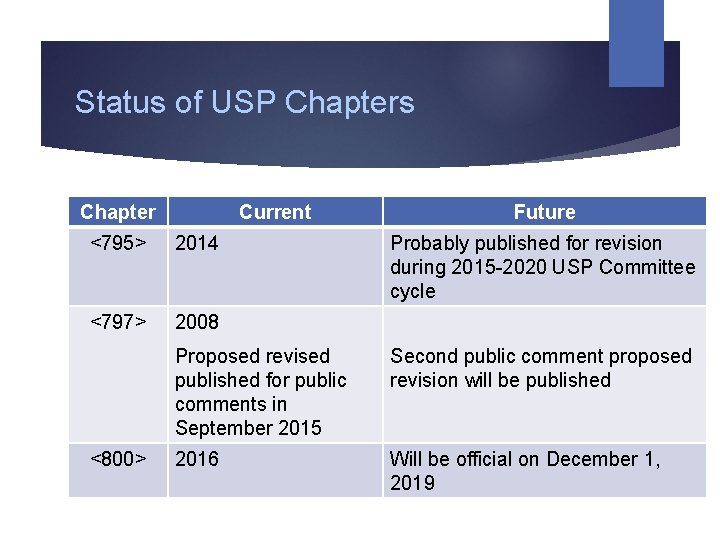
Status of USP Chapters Chapter Current <795> 2014 <797> 2008 <800> Future Probably published for revision during 2015 -2020 USP Committee cycle Proposed revised published for public comments in September 2015 Second public comment proposed revision will be published 2016 Will be official on December 1, 2019

Legal Status of USP Chapters Standards in USP-NF for compounded preparations may be enforced by States (as pharmacy practice/compounding is traditionally regulated by state boards of pharmacy) FDA (as compounded preparations remain subject to the adulteration and misbranding provisions of the FD&C Act which require conformance to certain USP-NF standards

Major Changes in Proposed <797> Removal Intent of hazardous drug section to Revise will direct you to <800> Change from three ingredient-based risk levels to two categories based on facility Segregated Compounding Area Cleanroom suite Limited BUD for Category 1 for any PEC Increased frequency of monitoring

Dedicated Compounding Room Massachusetts DCR Only allowed in institutions Only for non-hazardous, low-risk CSPs C-SCA with classified air Must be at least ISO 8

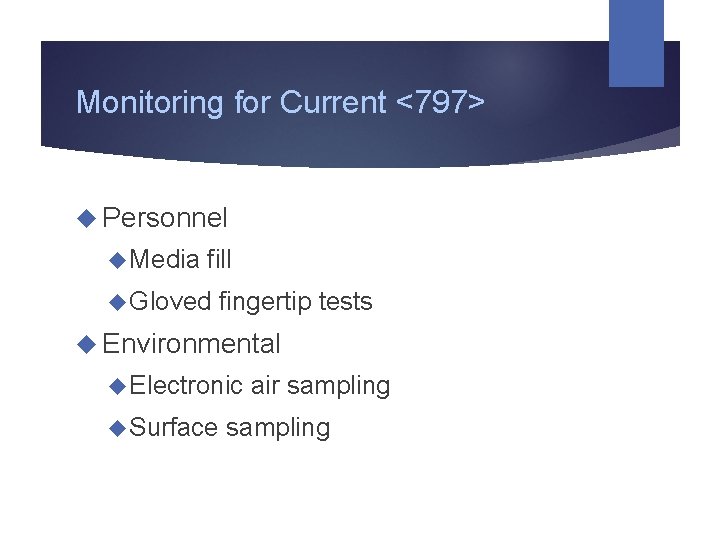
Monitoring for Current <797> Personnel Media fill Gloved fingertip tests Environmental Electronic Surface air sampling

Purpose of USP <800 Approximately 8 million workers are potentially exposed to hazardous drugs (HDs) each year <800> was developed to promote patient safety, worker safety, and environmental protection Defines practice and quality standards for handling HDs Builds on existing science, guidelines, and expertise

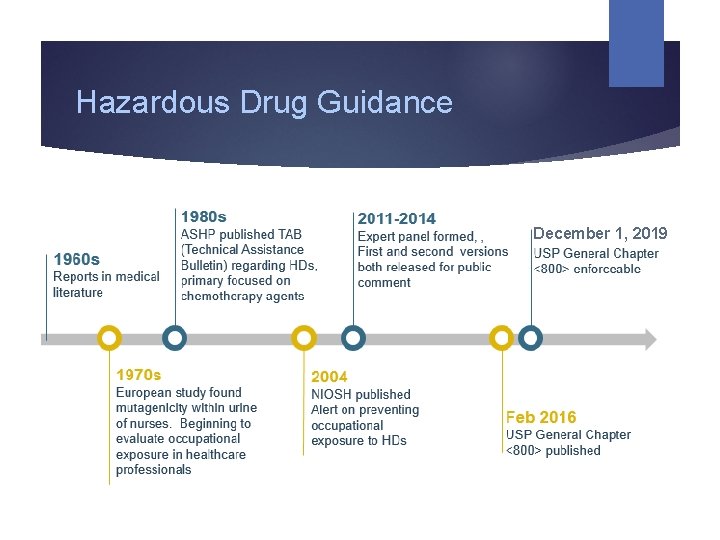
Hazardous Drug Guidance December 1, 2019

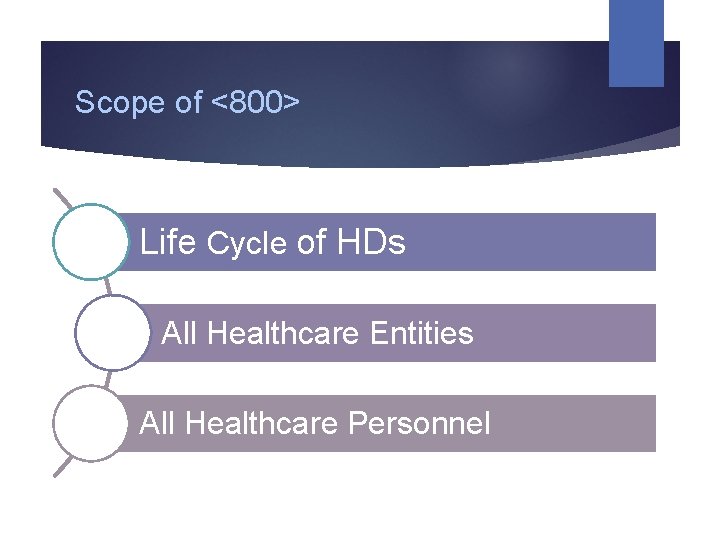
Scope of <800> Life Cycle of HDs All Healthcare Entities All Healthcare Personnel

What is a Hazardous Drug? Any drug identified by at least one of the following criteria Carcinogenicity Teratogenicity or other developmental toxicity Reproductive toxicity Organ toxicity at low doses Genotoxicity Structure and toxicity profile of new drug that mimics existing HD

Scope of <800> Receive Discard Transport Administer Store Mix

Official Date of USP <800> Official on December 1, 2019 This is a recent change Enforceable Federal agencies State agencies Accreditation organizations

Genesis of <800>

Elements of <800> Facilities PPE Hazard Communication Transport & Disposal Dispensing & Administration Compounding Cleaning Medical Surveillance

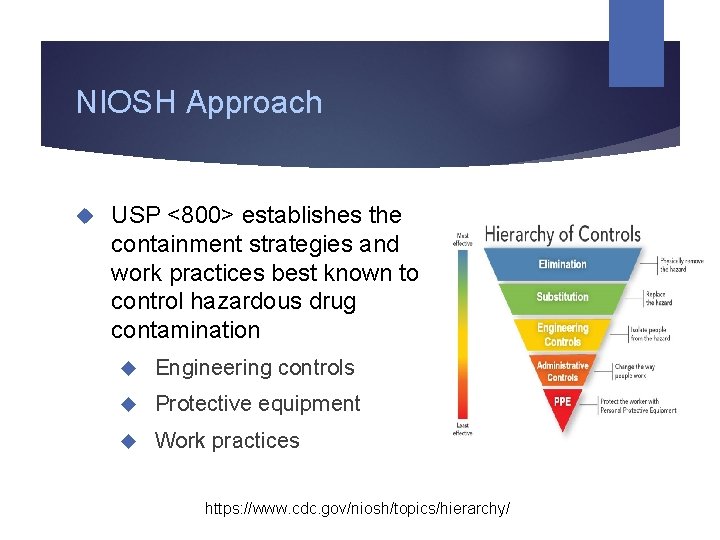
NIOSH Approach USP <800> establishes the containment strategies and work practices best known to control hazardous drug contamination Engineering controls Protective equipment Work practices https: //www. cdc. gov/niosh/topics/hierarchy/

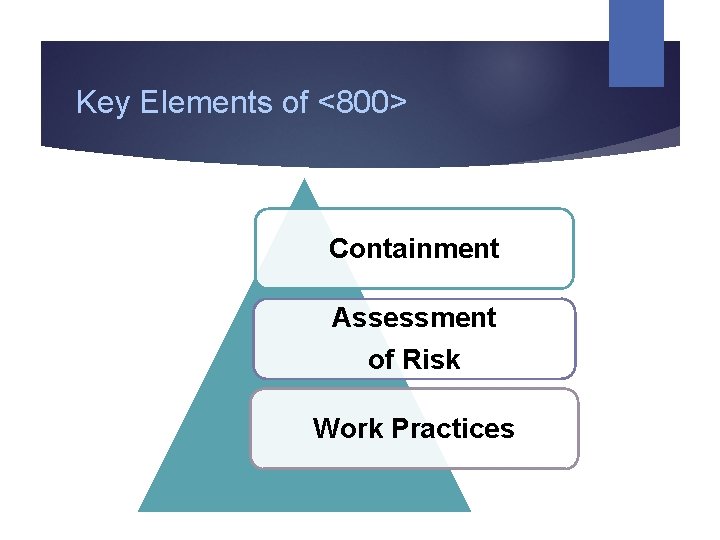
Key Elements of <800> Containment Assessment of Risk Work Practices

NIOSH List of Hazardous Drugs Hazardous to healthcare personnel Use of the list is required Different from EPA hazardous materials which are hazardous to the environment Tables 1, 2, and 3 Use of Table 5 Personal Protective Equipment (PPE) is not required, but provides a comprehensive list for policy development www. cdc. gov/niosh/docs/2016 -161/pdfs/2016 -161. pdf

What’s the status of your Assessment of Risk? A. Completed B. Identified list and working on Assessment C. Need to get working on this D. No need to do this since we don’t handle hazardous drugs

Your HD List Must contain all the HDs on the NIOSH list that you handle Must be specific to the dosage form level

Acknowledgement of Risk OSHA Hazard Communication Standard (HCS) is based on the concept that employees have both a need and a right to know the hazards and identifies the chemicals they are exposed to when working <800> Personnel of reproductive capability must confirm in writing that they understand the risks of handling HDs https: //www. pppmag. com/documents/V 8 N 10/CC/PDFs/Haz. Drug. Risk_Acknowledg. pdf

NIOSH List of Antineoplastics and Other Hazardous Drugs Antineoplastics Non-Antineoplastics Reproductive-Only Hazards

Your Options Handle all drugs and dosage forms with all containment and work practices listed in <800> Perform an Assessment of Risk to determine alternative containment strategies and work practices

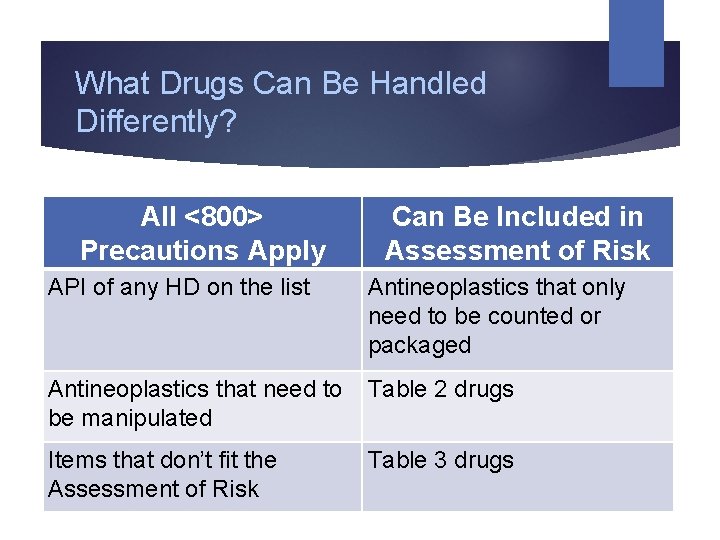
What Drugs Can Be Handled Differently? All <800> Precautions Apply Can Be Included in Assessment of Risk API of any HD on the list Antineoplastics that only need to be counted or packaged Antineoplastics that need to be manipulated Table 2 drugs Items that don’t fit the Assessment of Risk Table 3 drugs

Active Pharmaceutical Ingredient Any substance or mixture of substances intended to be used in the compounding of a drug preparation “Raw chemical” NOT finished dosage forms

Compliance with All <800> Elements Facilities Containment Primary Engineering Control (CPEC) Containment Secondary Engineering Control (C -SEC) Personal Protective Equipment (PPE) Work practices Policies and procedures Containment from receiving to dispensing Decontamination of work surfaces

OK to Consider for Assessment of Risk Antineoplastics that only need to be counted or packaged Non-antineoplastic meds (Table 2) Reproductive-only hazards (Table 3)

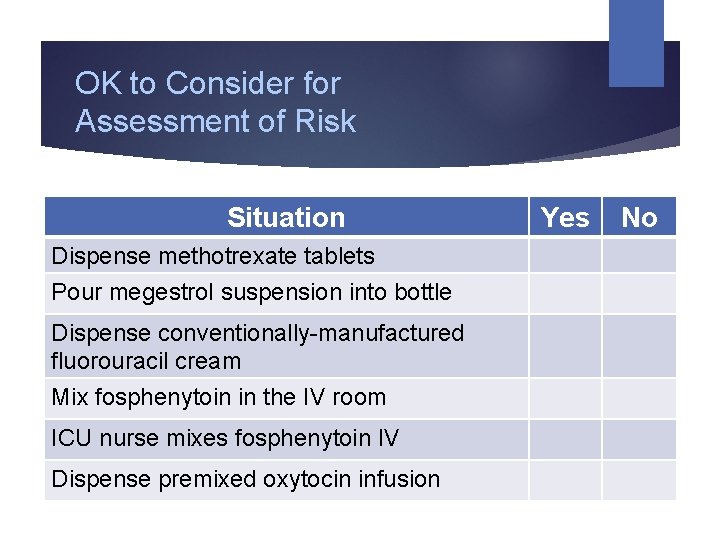
OK to Consider for Assessment of Risk Situation Dispense methotrexate tablets Pour megestrol suspension into bottle Dispense conventionally-manufactured fluorouracil cream Mix fosphenytoin in the IV room ICU nurse mixes fosphenytoin IV Dispense premixed oxytocin infusion Yes No

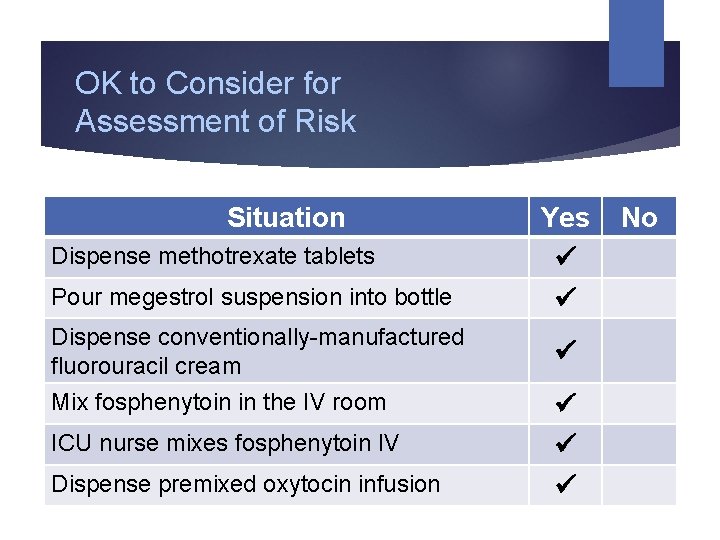
OK to Consider for Assessment of Risk Situation Dispense methotrexate tablets Pour megestrol suspension into bottle Dispense conventionally-manufactured fluorouracil cream Mix fosphenytoin in the IV room ICU nurse mixes fosphenytoin IV Dispense premixed oxytocin infusion Yes No

Alternative Strategy Examples Purchase unit-dose or unit-of-use Store in lidded bins Use closed system drug-transfer devices (CSTDs) Handle with chemo gloves Designate tackle boxes for transport

“Must” vs “Should” Must or shall = requirement Should = recommendation Use of CSTDs for compounding Wipe samples for environmental sampling Medical surveillance Photo courtesy of USP

Receiving and Storage HDs can be received in the same area as other drugs Should have a designated area Can be neutral/normal or negative pressure Cannot be positive pressure HDs (unless entity-exempt through the Assessment of Risk) Must be stored with proper containment

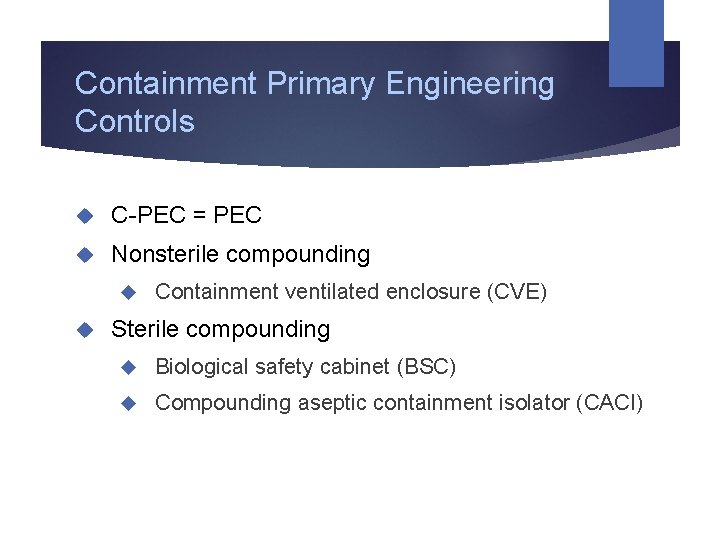
Containment Primary Engineering Controls C-PEC = PEC Nonsterile compounding Containment ventilated enclosure (CVE) Sterile compounding Biological safety cabinet (BSC) Compounding aseptic containment isolator (CACI)

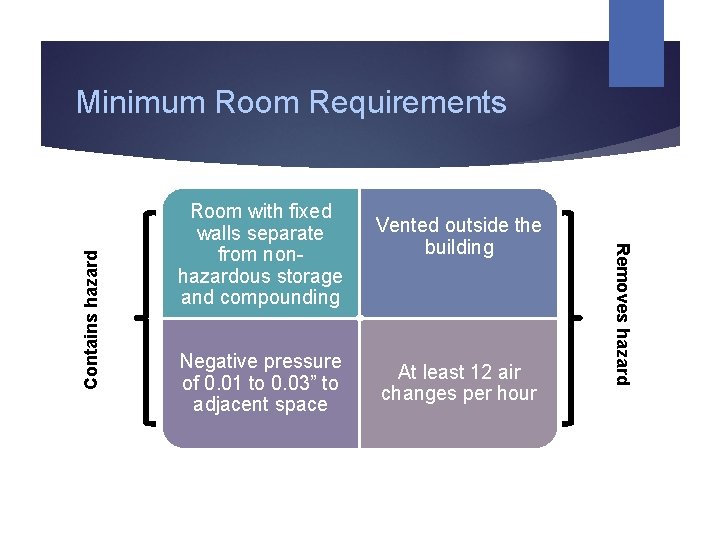
Room with fixed walls separate from nonhazardous storage and compounding Negative pressure of 0. 01 to 0. 03” to adjacent space Vented outside the building At least 12 air changes per hour Removes hazard Contains hazard Minimum Room Requirements

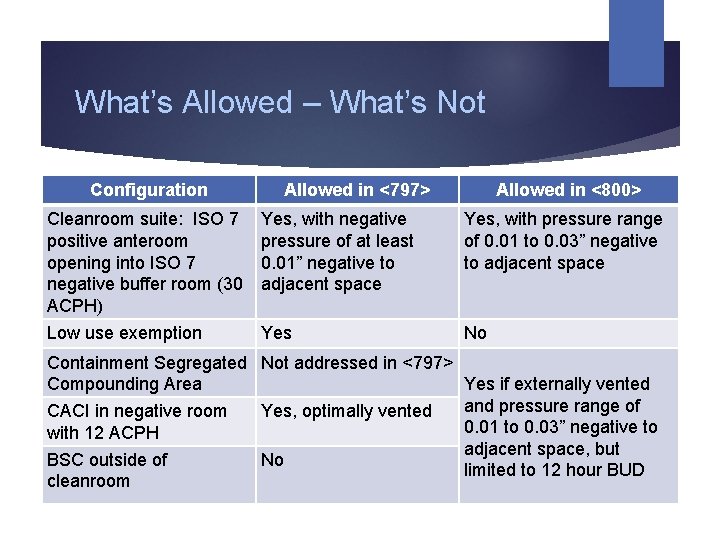
What’s Allowed – What’s Not Configuration Allowed in <797> Allowed in <800> Cleanroom suite: ISO 7 positive anteroom opening into ISO 7 negative buffer room (30 ACPH) Yes, with negative pressure of at least 0. 01” negative to adjacent space Yes, with pressure range of 0. 01 to 0. 03” negative to adjacent space Low use exemption Yes No Containment Segregated Not addressed in <797> Yes if externally vented Compounding Area and pressure range of CACI in negative room Yes, optimally vented 0. 01 to 0. 03” negative to with 12 ACPH adjacent space, but BSC outside of No limited to 12 hour BUD cleanroom

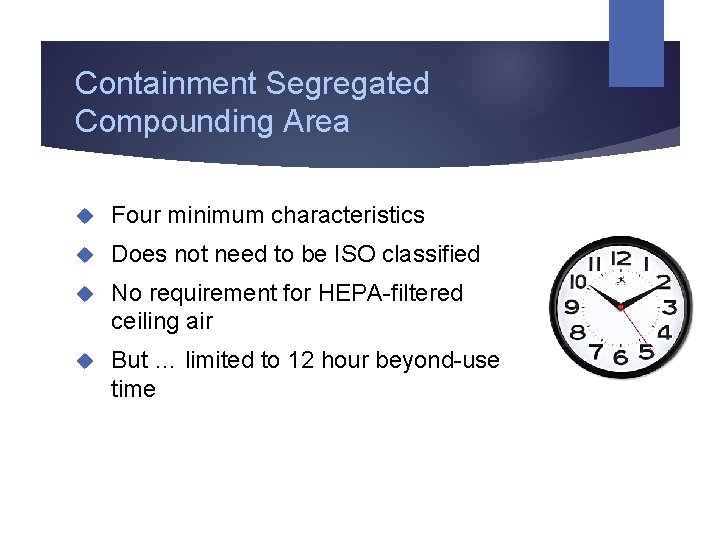
Containment Segregated Compounding Area Four minimum characteristics Does not need to be ISO classified No requirement for HEPA-filtered ceiling air But … limited to 12 hour beyond-use time

Closed System Drug-Transfer Devices (CSTDs) CSTDs mechanically prohibit the transfer of environmental contaminants into the system and the escape of hazardous drug or vapor Required for administration when the dosage form allows Recommended for use when compounding Photo courtesy of BD

Personal Protective Equipment Mask (to protect the preparation) E P P Hair covers d le e b s a s -u o e p r s e Shoe covers b Di t no n a c Gloves tested to ASTM D 6978 Gowns that are impervious, close in back, knit or elastic sleeves Respirator (to protect the compounder) Eye protection

Work Practices Policies and procedures Containment Outside of container once compound completed Waiting for patient pickup or transfer to administration area PPE Two pairs of shoe covers in negative space Outer pair removed prior to entering anteroom Decontamination procedures

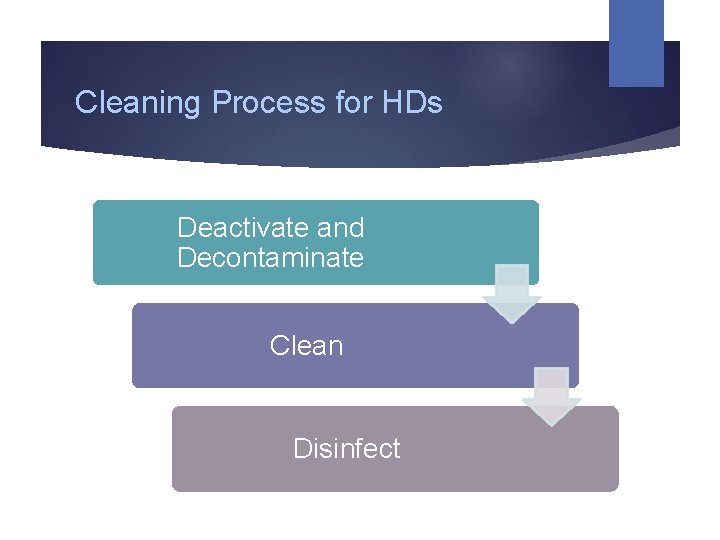
Cleaning Process for HDs Deactivate and Decontaminate Clean Disinfect

Cleaning Steps Function Deactivate and decontaminate Clean Disinfect Agent Properly-diluted EPAapproved oxidizer intended for use with HDs Germicidal detergent 70% isopropyl alcohol (sterile for sterile compounding)

Decontamination Under work tray of BSC or CAI Counting trays and spatulas Outside of containers Photo courtesy of Contec, Inc.

Resources for <797> USP website www. usp. org ASHP Sterile Compounding Resource Center

Resources for <800> USP <800> FAQs http: //www. usp. org/frequently-asked-questions/hazardousdrugs-handling-healthcare-settings www. readyfor 800. com One hour panel discussion (physician, nurse, pharmacist Short (~5 minute) videos targeted to specific audiences Ready for 800 checklist Perform an Assessment of Risk to Comply with USP <800> Pharmacy Purchasing and Products (www. pppmag. com), March 2017

References for <800> www. ashp. org www. hazmedsafety. com www. ons. org
