Using the NCDR Registries for Clinical Research John












- Slides: 32

Using the NCDR Registries for Clinical Research John C. Messenger MD, FACC, FSCAI Professor of Medicine Director, Cardiac Cath Labs and Cardiovascular ICU University of Colorado School of Medicine

John C. Messenger, MD Disclosures: • Philips Medical Systems, Institutional Grant Support • Chair, Science and Quality Oversight Committee, ACC National Cardiovascular Data Registry • Steering Committee, NCDR Reduce the Risk: PCI Bleed

Outline • NCDR at a Glance • Use of Artificial Intelligence to Improve Risk Models – Machine learning and AI research • FDA Collaboration for Post-Market Surveillance – Comparative effectiveness research • National Quality Campaign to Reduce Bleeding in PCI – Clinical quality assessment and improvement research

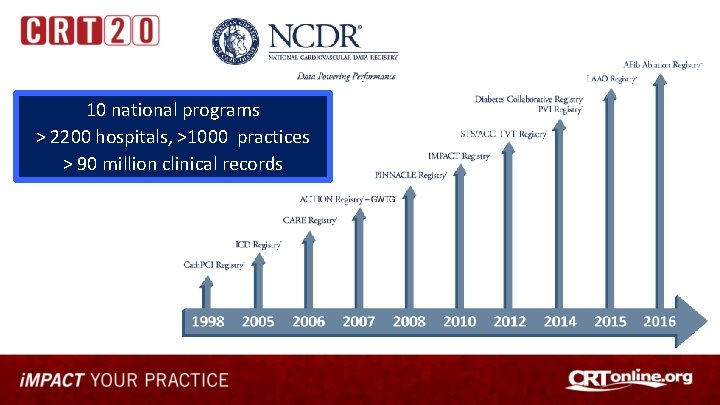
10 national programs > 2200 hospitals, >1000 practices > 90 million clinical records

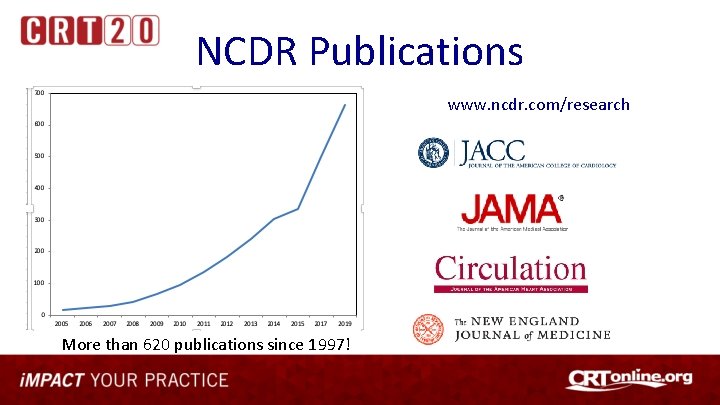
NCDR Publications www. ncdr. com/research More than 620 publications since 1997!

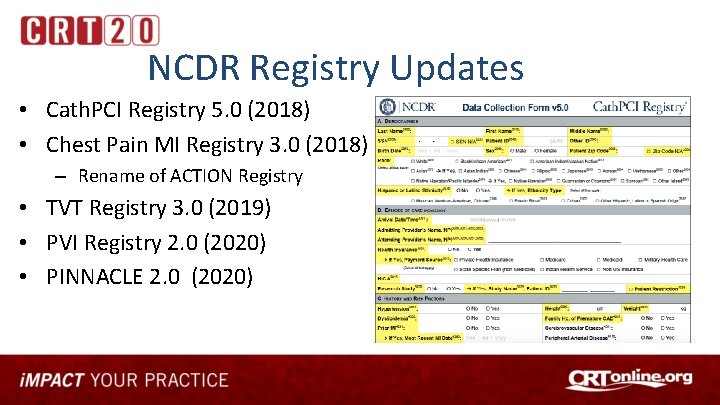
NCDR Registry Updates • Cath. PCI Registry 5. 0 (2018) • Chest Pain MI Registry 3. 0 (2018) – Rename of ACTION Registry • TVT Registry 3. 0 (2019) • PVI Registry 2. 0 (2020) • PINNACLE 2. 0 (2020)

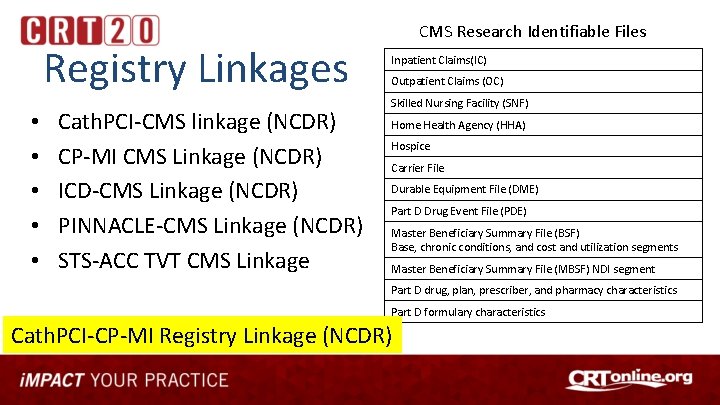
Registry Linkages • • • Cath. PCI-CMS linkage (NCDR) CP-MI CMS Linkage (NCDR) ICD-CMS Linkage (NCDR) PINNACLE-CMS Linkage (NCDR) STS-ACC TVT CMS Linkage CMS Research Identifiable Files Inpatient Claims(IC) Outpatient Claims (OC) Skilled Nursing Facility (SNF) Home Health Agency (HHA) Hospice Carrier File Durable Equipment File (DME) Part D Drug Event File (PDE) Master Beneficiary Summary File (BSF) Base, chronic conditions, and cost and utilization segments Master Beneficiary Summary File (MBSF) NDI segment Part D drug, plan, prescriber, and pharmacy characteristics Part D formulary characteristics Cath. PCI-CP-MI Registry Linkage (NCDR)

Outline • • NCDR at a glance Use of AI to improve risk models FDA Collaboration for Post-Market Surveillance National Quality Campaign to Reduce Bleeding in PCI


Use of AI to Improve Risk Models • Developed machine learning model for estimating patients’ preprocedural risk of AKI • Employed a generalized additive model (GAM) to accommodate potential nonlinear associations between contrast volume and AKI risk and the heterogeneity of this association among different risk groups Huang C et al. JAMA Network Open. 2019; 2(11)

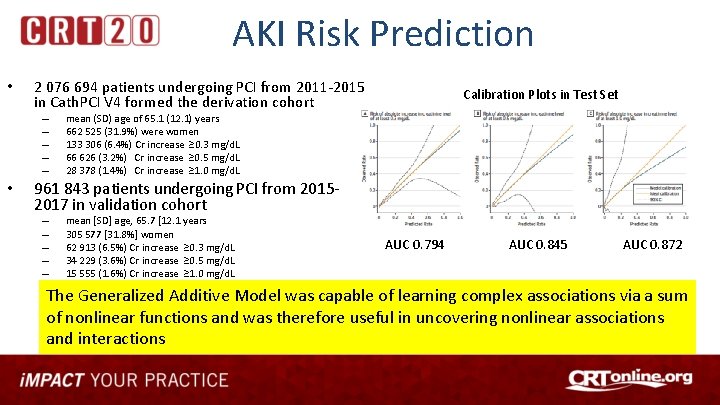
AKI Risk Prediction • 2 076 694 patients undergoing PCI from 2011 -2015 in Cath. PCI V 4 formed the derivation cohort – – – • Calibration Plots in Test Set mean (SD) age of 65. 1 (12. 1) years 662 525 (31. 9%) were women 133 306 (6. 4%) Cr increase ≥ 0. 3 mg/d. L 66 626 (3. 2%) Cr increase ≥ 0. 5 mg/d. L 28 378 (1. 4%) Cr increase ≥ 1. 0 mg/d. L 961 843 patients undergoing PCI from 20152017 in validation cohort – – – mean [SD] age, 65. 7 [12. 1 years 305 577 [31. 8%] women 62 913 (6. 5%) Cr increase ≥ 0. 3 mg/d. L 34 229 (3. 6%) Cr increase ≥ 0. 5 mg/d. L 15 555 (1. 6%) Cr increase ≥ 1. 0 mg/d. L AUC 0. 794 AUC 0. 845 AUC 0. 872 The Generalized Additive Model was capable of learning complex associations via a sum of nonlinear functions and was therefore useful in uncovering nonlinear associations and interactions

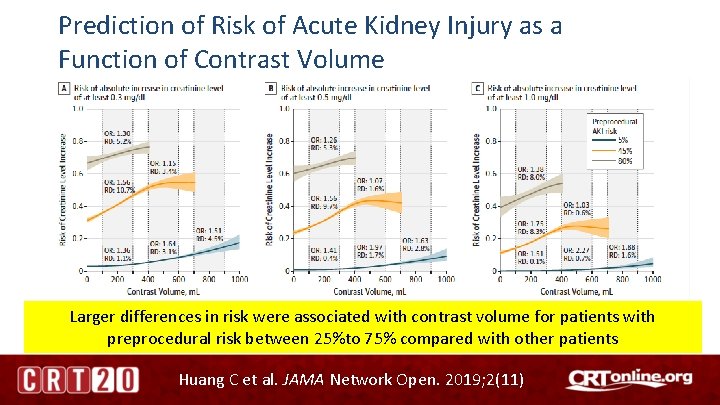
Prediction of Risk of Acute Kidney Injury as a Function of Contrast Volume Larger differences in risk were associated with contrast volume for patients with preprocedural risk between 25%to 75% compared with other patients Huang C et al. JAMA Network Open. 2019; 2(11)

Evaluation of Machine Learning using NCDR Data • 993, 905 patients in the NCDR Chest Pain. MI Registry hospitalized with AMI (mean age 64 ± 13 years, 34% women) between 1/2011 and 12/2016 • Developed and validated three machine learning models to predict in-hospital mortality after AMI – compared the performance characteristics with the current logistic regression (LR) model Khera R et al. bio. Rxiv 540369, 2019

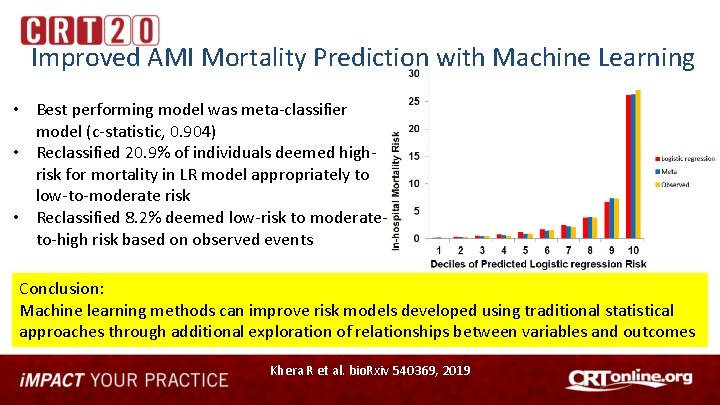
Improved AMI Mortality Prediction with Machine Learning • Best performing model was meta-classifier model (c-statistic, 0. 904) • Reclassified 20. 9% of individuals deemed highrisk for mortality in LR model appropriately to low-to-moderate risk • Reclassified 8. 2% deemed low-risk to moderateto-high risk based on observed events Conclusion: Machine learning methods can improve risk models developed using traditional statistical approaches through additional exploration of relationships between variables and outcomes Khera R et al. bio. Rxiv 540369, 2019

Outline • • NCDR at a glance Use of AI to improve risk models FDA Collaboration for Post-Market Surveillance National Quality Campaign to Reduce Bleeding in PCI

Post Market Approval Device Surveillance Using NCDR Data • Funding for this research was provided by a Center of Excellence in Regulatory Science and Innovation (CERSI) grant to Yale University and Mayo Clinic from the FDA (U 01 FD 005938) Dhruva SS et al. JAMA 2020

Study Aims 1) Assess MCS device utilization over time among patients with AMI complicated by cardiogenic shock (AMI-CS) undergoing PCI 2) Apply propensity score matching to examine clinical outcomes among patients receiving Impella® or IABP A. Primary effectiveness outcome: in-hospital death B. Primary safety outcome: in-hospital major bleeding

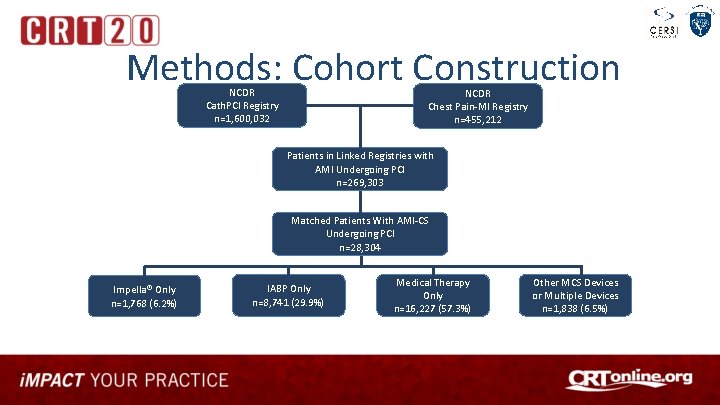
Methods: Cohort Construction NCDR Cath. PCI Registry n=1, 600, 032 NCDR Chest Pain-MI Registry n=455, 212 Patients in Linked Registries with AMI Undergoing PCI n=269, 303 Matched Patients With AMI-CS Undergoing PCI n=28, 304 Impella® Only n=1, 768 (6. 2%) IABP Only n=8, 741 (29. 9%) Medical Therapy Only n=16, 227 (57. 3%) Other MCS Devices or Multiple Devices n=1, 838 (6. 5%)

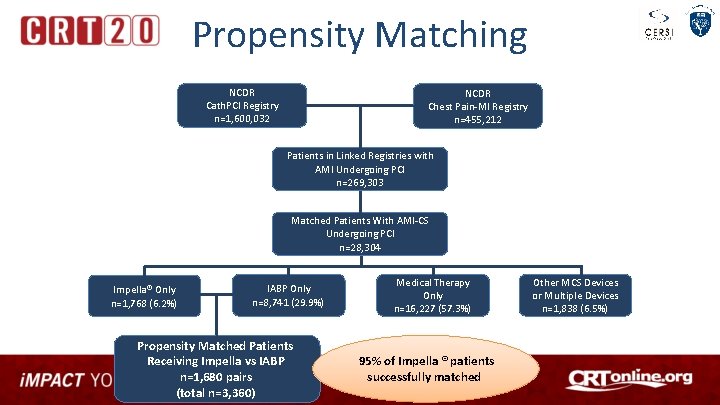
Propensity Matching NCDR Cath. PCI Registry n=1, 600, 032 NCDR Chest Pain-MI Registry n=455, 212 Patients in Linked Registries with AMI Undergoing PCI n=269, 303 Matched Patients With AMI-CS Undergoing PCI n=28, 304 Impella® Only n=1, 768 (6. 2%) IABP Only n=8, 741 (29. 9%) Propensity Matched Patients Receiving Impella vs IABP n=1, 680 pairs (total n=3, 360) Medical Therapy Only n=16, 227 (57. 3%) 95% of Impella ® patients successfully matched Other MCS Devices or Multiple Devices n=1, 838 (6. 5%)

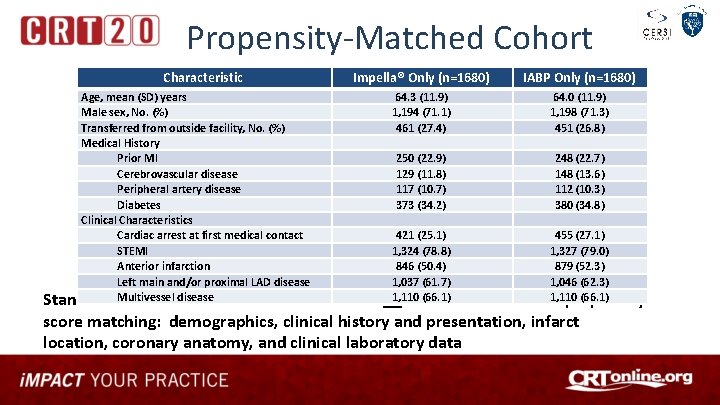
Propensity-Matched Cohort Characteristic Impella® Only (n=1680) Age, mean (SD) years Male sex, No. (%) Transferred from outside facility, No. (%) Medical History Prior MI Cerebrovascular disease Peripheral artery disease Diabetes Clinical Characteristics Cardiac arrest at first medical contact STEMI Anterior infarction Left main and/or proximal LAD disease Multivessel disease Standardized mean difference was <0. 10 for IABP Only (n=1680) 64. 3 (11. 9) 1, 194 (71. 1) 461 (27. 4) 64. 0 (11. 9) 1, 198 (71. 3) 451 (26. 8) 250 (22. 9) 129 (11. 8) 117 (10. 7) 373 (34. 2) 248 (22. 7) 148 (13. 6) 112 (10. 3) 380 (34. 8) 421 (25. 1) 455 (27. 1) 1, 324 (78. 8) 1, 327 (79. 0) 846 (50. 4) 879 (52. 3) 1, 037 (61. 7) 1, 046 (62. 3) 1, 110 (66. 1) all 77 variables used for propensity score matching: demographics, clinical history and presentation, infarct location, coronary anatomy, and clinical laboratory data

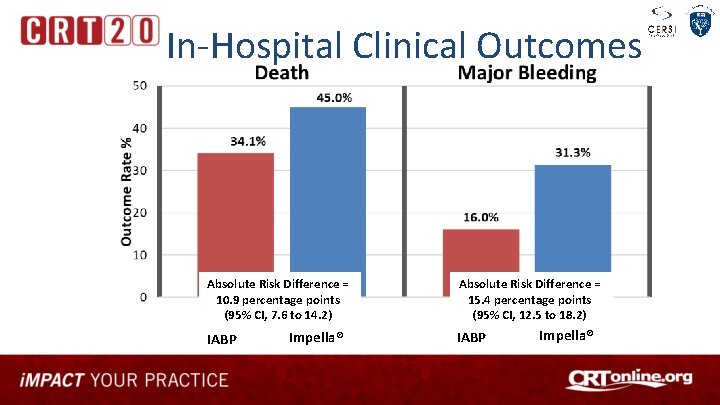
In-Hospital Clinical Outcomes Absolute Risk Difference = 10. 9 percentage points (95% CI, 7. 6 to 14. 2) IABP Impella® Absolute Risk Difference = 15. 4 percentage points (95% CI, 12. 5 to 18. 2) IABP Impella®

Study Conclusions This large, nationally representative study of patients with AMI-CS undergoing PCI using NCDR Data from two linked registries demonstrated: – A significant 2. 5 -fold increase in the utilization of Impella® devices over time – Use of Impella® was associated with significantly higher rates of in-hospital mortality and bleeding, regardless of timing of device placement • Outcomes associated with MCS devices may differ in real-world settings as compared to performance within RCT settings • Better evidence and guidance is needed regarding the optimal management of patients with AMI-CS

Outline • • NCDR at a glance Use of AI to improve risk models FDA Collaboration for Post-Market Surveillance National Quality Campaign to Reduce Bleeding in PCI

The Campaign Goal MOTIVATE WIDESPREAD ADOPTION OF EVIDENCE -BASED PRACTICES DECREASE VARIATION IN CARE DECREASE THE INCIDENCE OF BLEEDING

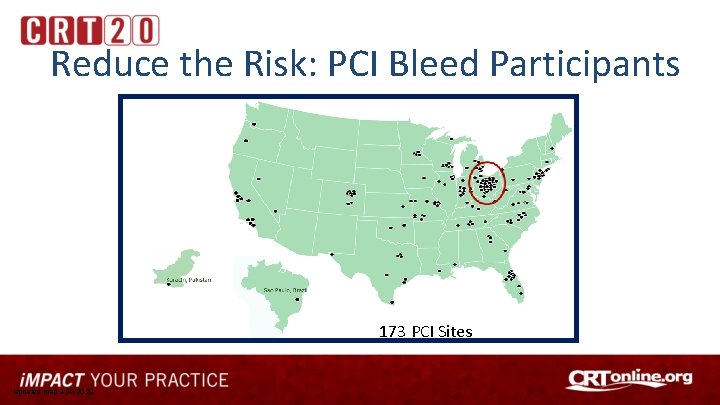
Reduce the Risk: PCI Bleed Participants 173 PCI Sites Updated map 2 -10 -2020

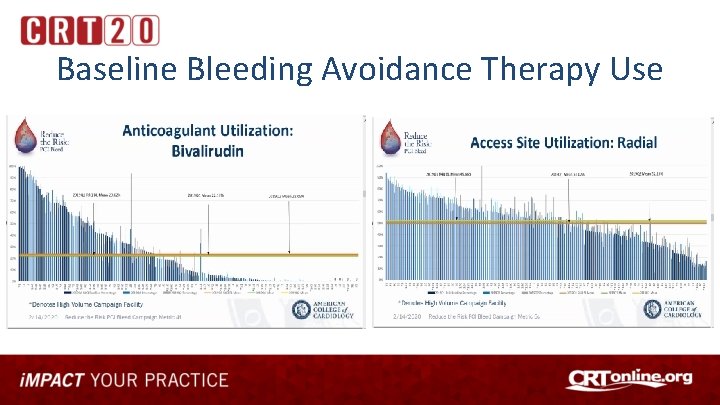
Baseline Bleeding Avoidance Therapy Use

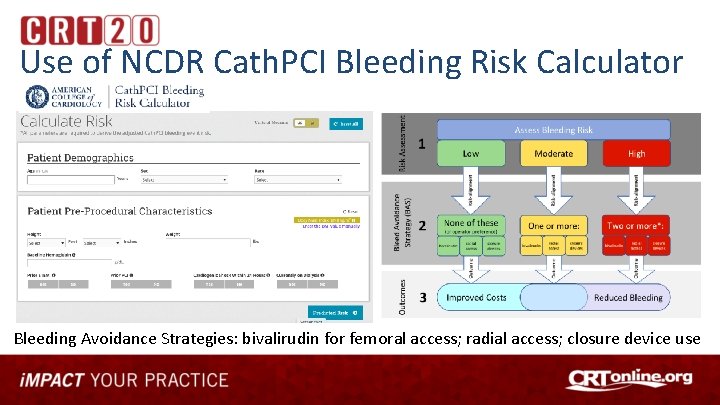
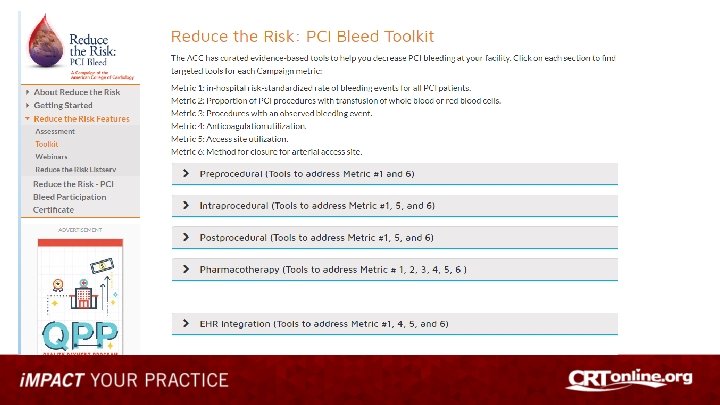
Investigating Use of NCDR Tools For Quality Improvement • Reduce the Risk: PCI Bleed Quality Campaign – focus on minimizing PCI-associated bleeding risks and reducing mortality through adoption of risk assessment tools and use of evidence-based best practices • • Baseline Performance Dashboards provided from each site’s Cath. PCI Data Use NCDR Bleeding Risk Calculator at Point of Care to assess bleeding risk Implement best practices and bleeding avoidance therapies based on risk Re-evaluate performance using before and after design using Dashboard

Use of NCDR Cath. PCI Bleeding Risk Calculator Bleeding Avoidance Strategies: bivalirudin for femoral access; radial access; closure device use


Study Plan: Re-evaluate Outcomes and Processes of Care Post Campaign

Conclusions • NCDR research covers a broad spectrum of cardiovascular patients and treatments that now allow long term follow up • NCDR registry data are now being used with machine learning techniques and AI to further improve risk models and risk prediction=>precision care • Data from NCDR linked registries recently used to evaluate device performance (Impella and IABP) in cardiogenic shock for post-market surveillance in conjunction with the FDA – Evaluate real-world use and outcomes of technologies/treatments • NCDR data is being used to benchmark care and evaluate the impact of a clinical toolkit to reduce bleeding around the time of PCI as a National Quality Campaign

Thank You John. Messenger@ucdenver. edu Pike’s Peak, Colorado