Using PKPD Modeling to Simulate Impact of Manufacturing
















- Slides: 41

Using PK/PD Modeling to Simulate Impact of Manufacturing Process Variability Alan Hartford Agensys Tim Schofield Biologics Consulting Group, Inc. The 32 nd Annual Midwest Biopharmaceutical Statistics Workshop May 18 – 20, 2009, Muncie, Indiana

Introduction n n The manufacturer has the responsibility of keeping manufacturing process variability of the dose in control. One method for assuring that a product, after a reformulation, is viable is to perform a clinical trial showing bioequivalence (BE) of exposure endpoints.

Investigate with Modeling n n PK/PD models can be used to simulate the impact of variations of dose on the response cascade of dose → exposure → pharmacodynamics → clinical outcome These models can address the appropriateness of n n the BE study choice of bounds Specific information from clinical development is needed as input for this PK/PD modeling. Important information from nonclinical development can also be incorporated to save clinical resources.

Outline n n n n Introduction Bioequivalence (BE) bounds PK/PD Models Predict effect of process variation on clinical outcome Required clinical information Collaborative modeling nonclinical/clinical Summary

Current Practice n n When a new formulation is developed, the current practice is to perform a clinical study to show the new formulation is “bioequivalent” to a previously studied formulation. This allows for inference of conclusions from earlier studies for the new formulation. n i. e. , efficacy results from a Ph III study can be inferred to a new formulation

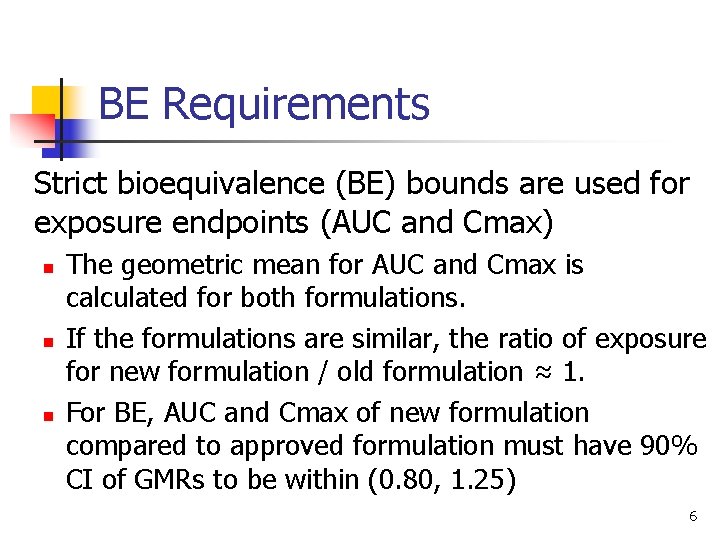
BE Requirements Strict bioequivalence (BE) bounds are used for exposure endpoints (AUC and Cmax) n n n The geometric mean for AUC and Cmax is calculated for both formulations. If the formulations are similar, the ratio of exposure for new formulation / old formulation ≈ 1. For BE, AUC and Cmax of new formulation compared to approved formulation must have 90% CI of GMRs to be within (0. 80, 1. 25) 6

BE Requirements (cont. ) n n This strict BE requirement is standard for many clinical comparisons (e. g. , interaction studies, elderly/young studies, insufficiency studies) But (0. 80, 1. 25) may not be appropriate for clinical reasons (0. 80, 1. 25) is standard for when no clinical justification can be given for other bounds If victim drug has wide therapeutic window, then wider bounds are appropriate 7

BE Requirements (cont. ) n n For drug interaction studies, FDA suggests that boundaries can be justified by a sponsor based on population average dose, concentration-response relationships, PK/PD models, or other So the onus is on the sponsor to justify other comparability bounds FDA Guidance: Drug Interaction Studies--Study Design, Data Analysis, and Implications for Dosing and Labeling (draft 2006)

Example of Using Alternate Comparability Bounds n In the case of testing if a new antibody has an effect on the exposure of a standard of care chemotherapy n n n Not ethical to sample many patients with cancer for this interaction trial Variability of the chemo (AUC, Cmax) was high FDA accepted plan with small N which required GMR to be within (0. 80, 1. 25) but that the 90% CI to be within (0. 70, 1. 43)

FDA Guidance to Clinical n n FDA Guidance for some studies (e. g. , interaction studies) allows for some leeway for sponsor to clinically justify alternative bounds PK/PD modeling can be used to justify different bounds for comparing formulations 10

Modeling & Simulation n PK/PD M&S and Clinical Trial Simulation can provide insight to n n n Effect of variation in dose on exposure Effect of variation in exposure on PD Effect of PD on clinical endpoint 11

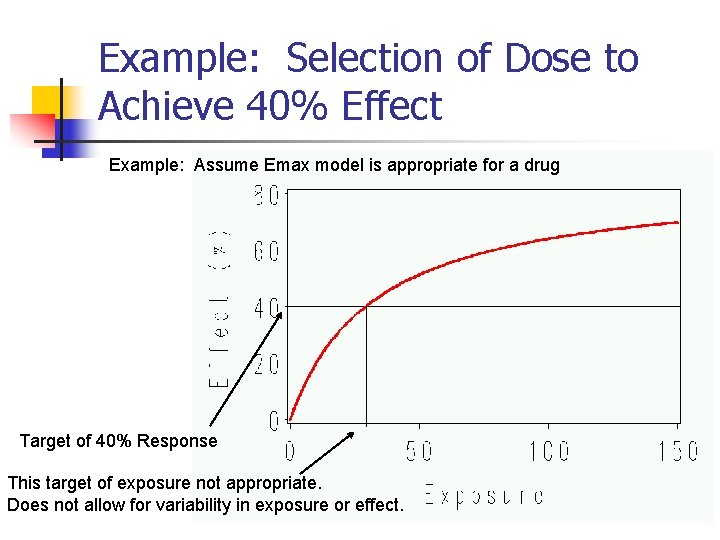
Example: Selection of Dose to Achieve 40% Effect Example: Assume Emax model is appropriate for a drug Target of 40% Response This target of exposure not appropriate. Does not allow for variability in exposure or effect. 12

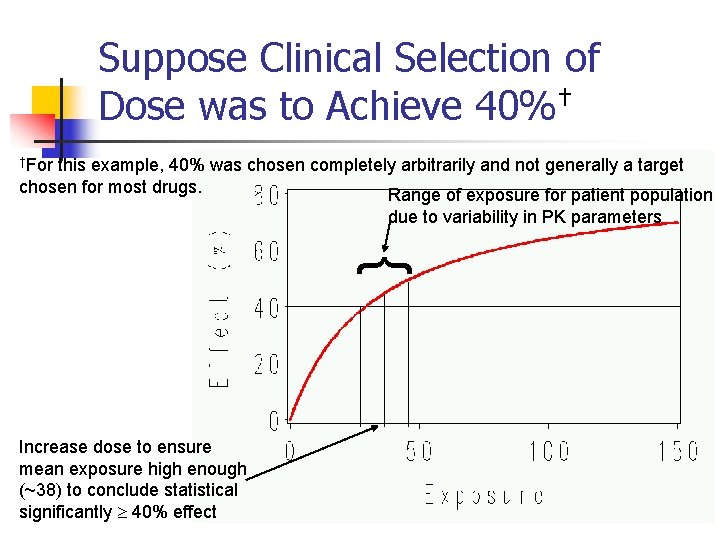
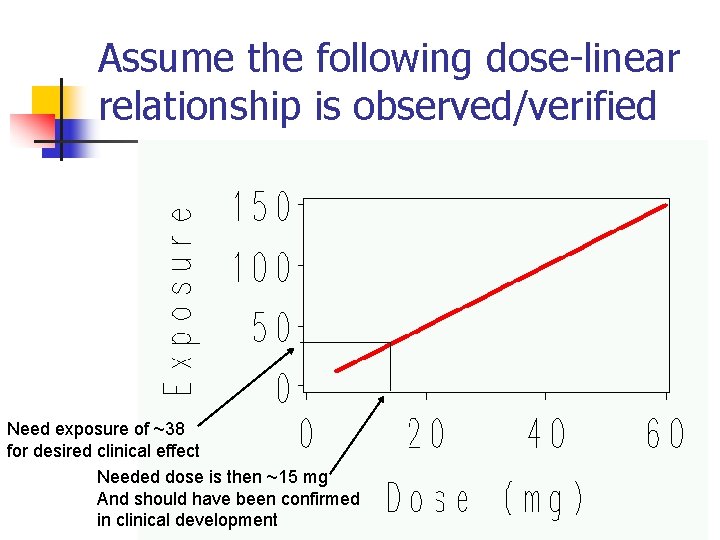
Suppose Clinical Selection of Dose was to Achieve 40%† †For this example, 40% was chosen completely arbitrarily and not generally a target chosen for most drugs. Range of exposure for patient population due to variability in PK parameters { Increase dose to ensure mean exposure high enough (~38) to conclude statistical significantly 40% effect 13

Assume the following dose-linear relationship is observed/verified Need exposure of ~38 for desired clinical effect Needed dose is then ~15 mg And should have been confirmed in clinical development 14

Manufacturing Variability n n Every manufacturing process has specification limits Product from this process is allowed to vary within these limits In this manner, the dosage of drug is not constant across a batch The effect of the manufacturing variability is what we need to understand

Incorporating Manufacturing Variability To determine effect of manufacturing variability on the sequence of Dose: Exposure: Response Perform simulations 1. Assume manufacturing variability limits 2. Using dose linear relationship and incorporating PK model with inter-subject variability, determine effect of additional variability due to manufacturing process on exposure 3. Using PK/PD model (e. g. , Emax), determine effect of compounded variability in exposure in step 2 on clinical or PD effect 16

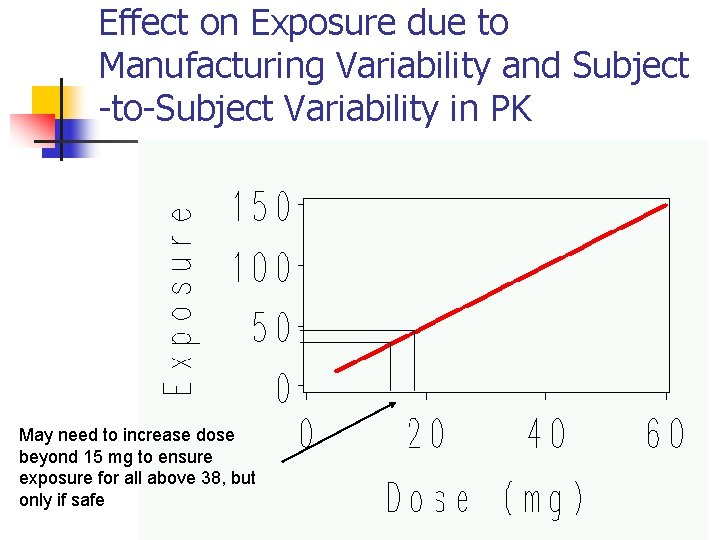
Effect on Exposure due to Manufacturing Variability and Subject -to-Subject Variability in PK May need to increase dose beyond 15 mg to ensure exposure for all above 38, but only if safe 17

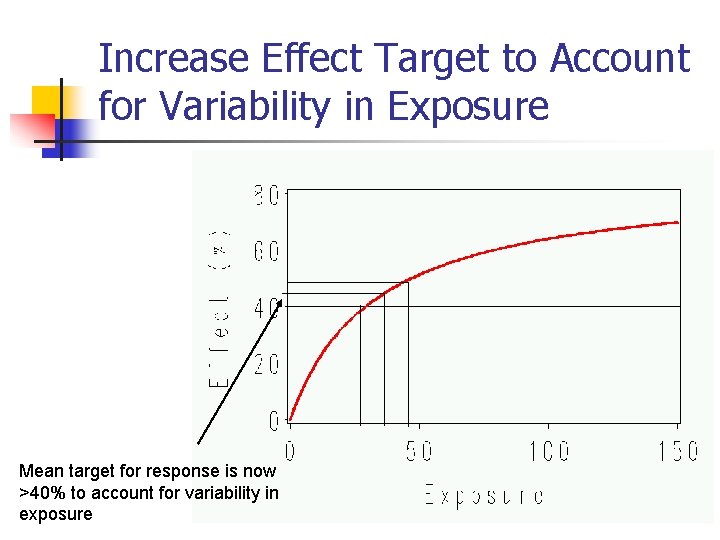
Increase Effect Target to Account for Variability in Exposure Mean target for response is now >40% to account for variability in exposure 18

Limits for Effect n n n Note that the 40% effect size was determined from Ph III development and not an effect size targeted in earlier studies Likewise, an upper limit for effect to be determined by the safety profile observed throughout clinical development Simulations using PK/PD models will help to determine acceptable limits of manufacturing variability 19

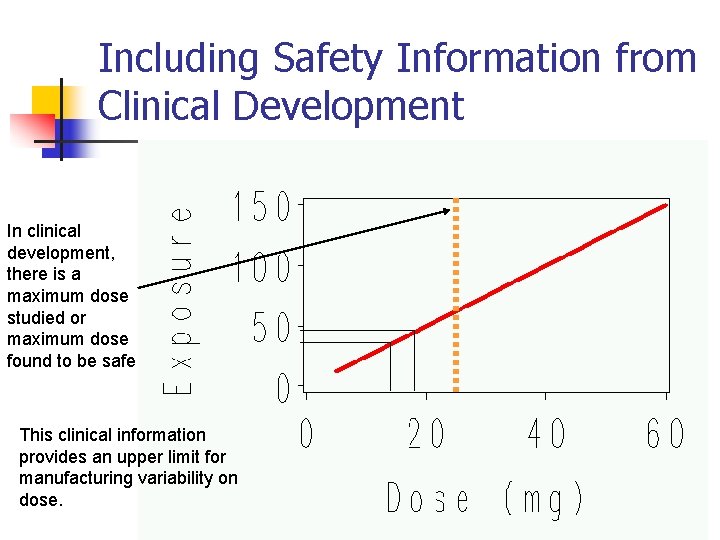
Including Safety Information from Clinical Development In clinical development, there is a maximum dose studied or maximum dose found to be safe This clinical information provides an upper limit for manufacturing variability on dose. 20

Required Clinical Information n The information needed from clinical development includes n n Upper limit on exposure due to safety Target response for efficacy

Required Clinical Information (cont. ) n Additionally, clinical information is needed to build the PK/PD model n n Need to sample responses across wide range of exposure values to understand what model is appropriate Note that this can be at odds with goals of adaptive designs

Dose-Exposure Relationship n n n Earlier, we assumed a linear doseexposure relationship However, this relationship might not be known for patients for a new formulation Nonclinical and preclinical modeling could be used to provide this information

Additional Modeling Opportunities n n delete Modeling approaches are used widely across drug development These different modeling efforts can be linked across nonclinical and clinical

delete Expanded Problem statement n How can nonclinical development collaborate with clinical development to demonstrate that a manufacturing process is delivering product to the patient that is safe and effective?

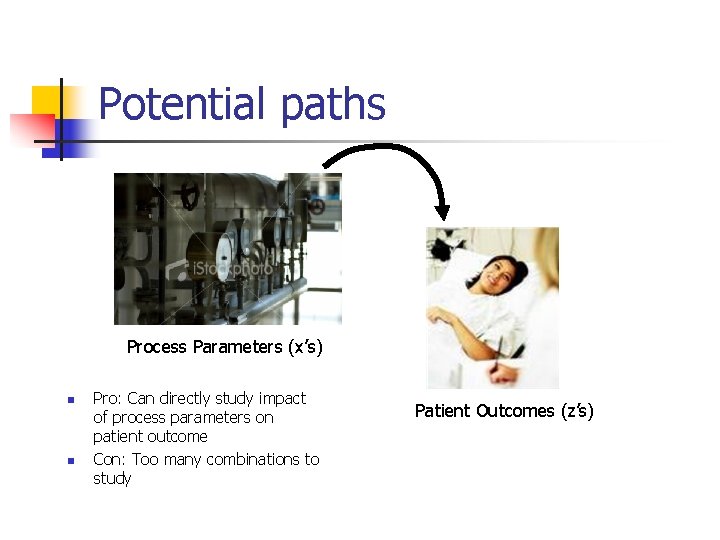
Potential paths Process Parameters (x’s) n n Pro: Can directly study impact of process parameters on patient outcome Con: Too many combinations to study Patient Outcomes (z’s)

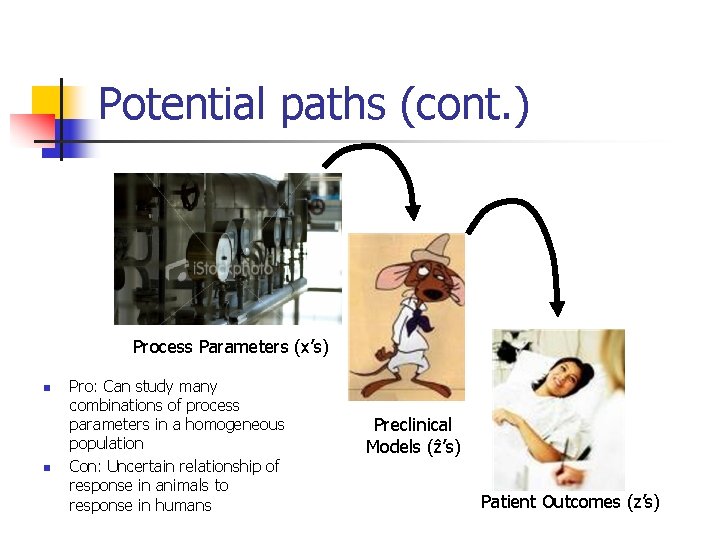
Potential paths (cont. ) Process Parameters (x’s) n n Pro: Can study many combinations of process parameters in a homogeneous population Con: Uncertain relationship of response in animals to response in humans Preclinical Models (ẑ’s) Patient Outcomes (z’s)

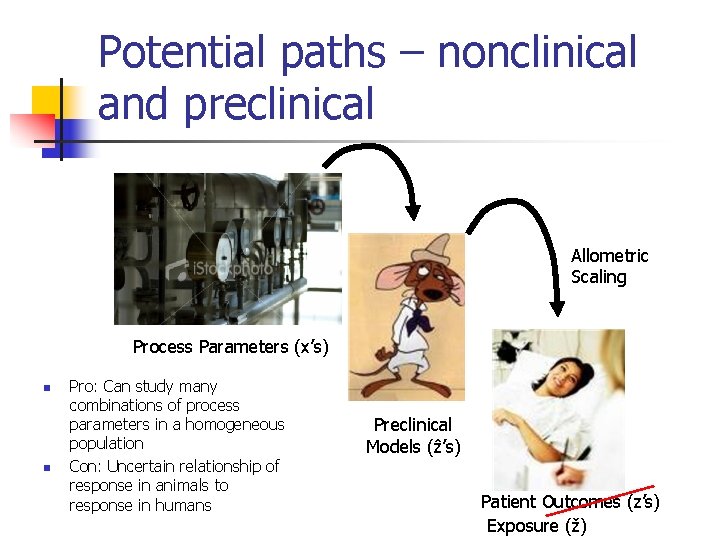
Potential paths – nonclinical and preclinical Allometric Scaling Process Parameters (x’s) n n Pro: Can study many combinations of process parameters in a homogeneous population Con: Uncertain relationship of response in animals to response in humans Preclinical Models (ẑ’s) Patient Outcomes (z’s) Exposure (ž)

Potential paths – nonclinical Process Parameters (x’s) n n Pro: Can study many combinations of process parameters in vitro Con: Less certain relationship of response in vitro to response in humans Quality Attributes (y’s) Patient Outcome (z’s)

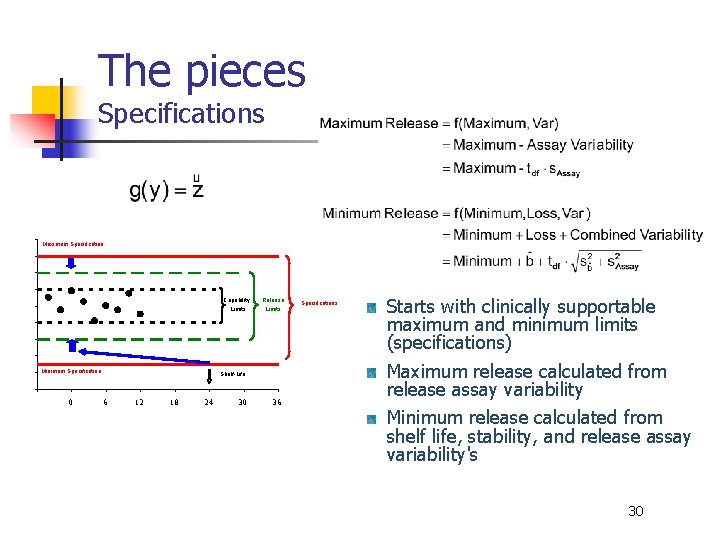
The pieces Specifications Maximum Specification Minimum Specification -6 0 Capability Release Limits Shelf-Life 6 12 18 24 30 36 Specifications Starts with clinically supportable maximum and minimum limits (specifications) Maximum release calculated from release assay variability Minimum release calculated from shelf life, stability, and release assay variability's 30

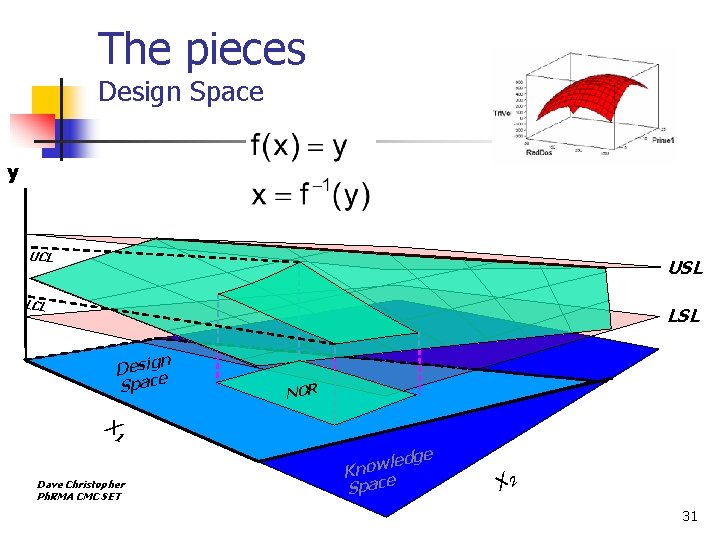
The pieces Design Space y UCL USL LCL LSL n Desig Space NOR X 1 Dave Christopher Ph. RMA CMC SET ledg w o n K Space e X 2 31

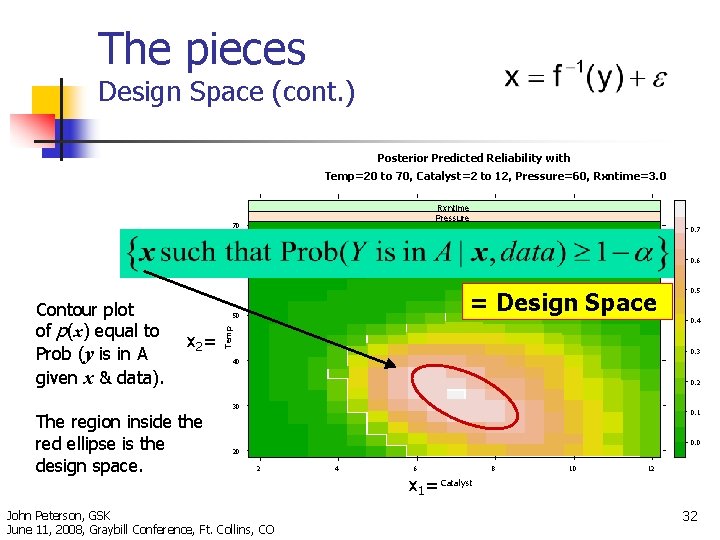
The pieces Design Space (cont. ) Posterior Predicted Reliability with Temp=20 to 70, Catalyst=2 to 12, Pressure=60, Rxntime=3. 0 Rxntime Pressure 70 0. 7 0. 6 60 = Design Space 50 x 2 = 0. 4 Temp Contour plot of p(x) equal to Prob (y is in A given x & data). 0. 3 40 0. 2 30 The region inside the red ellipse is the design space. 0. 5 0. 1 0. 0 20 2 John Peterson, GSK June 11, 2008, Graybill Conference, Ft. Collins, CO 4 6 8 10 12 x 1= Catalyst 32

The pieces IVIVC n An in-vitro in-vivo correlation (IVIVC) has been defined by the FDA as “a predictive mathematical model describing the relationship between an in-vitro property of dosage form and an in-vivo response” n n n Main objective is to serve as a surrogate for in vivo bioavailability and to support biowaivers Might also be used to bridge in vitro and in vivo activity along the pathway from manufacturing process to patient outcome IVIV relationship (IVIVR) more appropriate to the goal – g(y)=ž

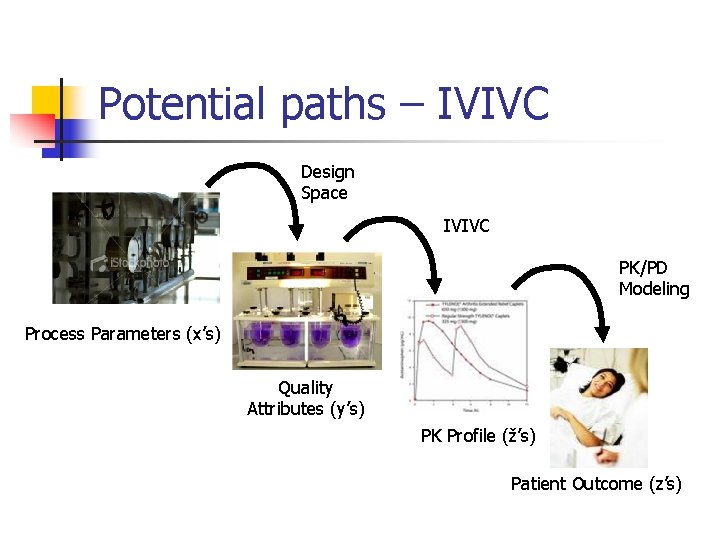
Potential paths – IVIVC Design Space IVIVC PK/PD Modeling Process Parameters (x’s) Quality Attributes (y’s) PK Profile (ž’s) Patient Outcome (z’s)

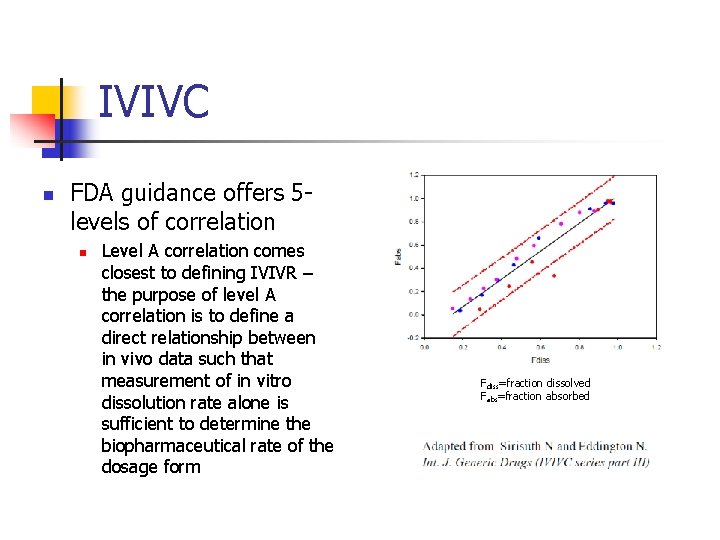
IVIVC n FDA guidance offers 5 levels of correlation n Level A correlation comes closest to defining IVIVR – the purpose of level A correlation is to define a direct relationship between in vivo data such that measurement of in vitro dissolution rate alone is sufficient to determine the biopharmaceutical rate of the dosage form Fdiss=fraction dissolved Fabs=fraction absorbed

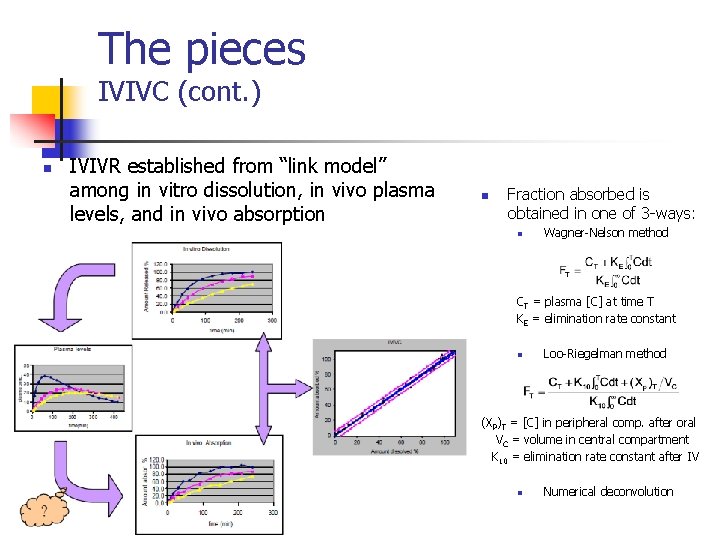
The pieces IVIVC (cont. ) n IVIVR established from “link model” among in vitro dissolution, in vivo plasma levels, and in vivo absorption n Fraction absorbed is obtained in one of 3 -ways: n Wagner-Nelson method CT = plasma [C] at time T KE = elimination rate constant n Loo-Riegelman method (XP)T = [C] in peripheral comp. after oral VC = volume in central compartment K 10 = elimination rate constant after IV n Numerical deconvolution

The Full Cascade of Information n n Processing Parameters (x’s) Quality Attributes (y’s) PK (exposure) Parameters (ž’s) PD or Clinical Outcome (z’s)

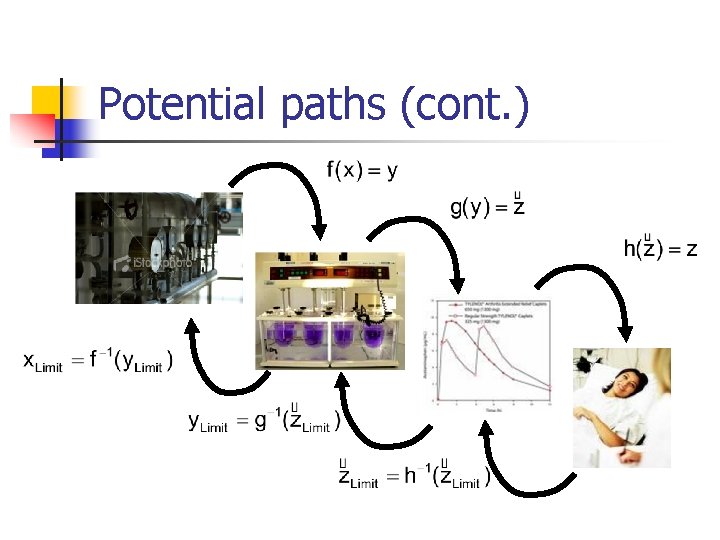
Potential paths (cont. )

Summary n n A process has been outlined for using information from different stages of drug development to determine process limits Process will inform decision about needing additional clinical trials for new formulations

Summary (cont. ) n Clinical information is needed for successful modeling n n Target for efficacy Safety In total, therapeutic window IVIVC or IVIVR models needed to inform about exposure

Summary (cont. ) n n Both PK/PD Modeling and IVIVC modeling are time-consuming and tedious and must be integrated early into development Designs of clinical trials must be designed so that information needed for building models is available