Using PETCT in Prostate Cancer Insert facilitypresenter information









- Slides: 23

Using PET/CT in Prostate Cancer Insert facility/presenter information here

Legal Disclaimer These materials were prepared in good faith by MITA as a service to the profession and are believed to be reliable based on current scientific literature. The materials are for educational purposes only and do not replace either the need for individualized patient diagnosis and treatment planning by qualified physicians based on existing good practices or the need for implementation by qualified radiologists or other qualified healthcare practitioners. Neither MITA nor its members are responsible for any diagnostic or treatment outcomes. MITA, its members, and contributors do not assume any responsibility for the user’s compliance with applicable laws and regulations. MITA does not endorse the proprietary products or processes of any one company.

Purpose and Audience Provide urologists, surgeons, medical oncologists, radiation oncologists and other prostate cancer specialists with the following information to aid in the diagnosis and management of prostate cancer patients § A summary of PET radiopharmaceuticals that are FDAapproved to image prostate cancer § NCCN Practice Guidelines updates for using PET/CT in prostate cancer § Recent Report from the National Oncologic PET Registry (NOPR) § Impact on patient management

PET/CT in Prostate Cancer PET/CT plays an important role in the evaluation of prostate cancer on many levels § Detecting metastatic disease § Restaging § Biochemical relapse post-radical therapy § A biochemical relapse is one where, after completing treatment (e. g. , prostatectomy), PSA begins rising again but the cancer cannot (yet) be detected by CT or MRI § Treatment monitoring § Use for primary staging generally limited to only high-risk disease Jadvar H. J Nucl Med 2013; 54(10): 1685 -1688

FDA-Approved PET Radiopharmaceuticals for Prostate Cancer § Tracers that image metabolism § C-11 choline and F-18 fluciclovine § Used to localize disease recurrence in men who have rising PSA and inconclusive conventional imaging § F-18 FDG § Used typically in patients with late-stage recurrent prostate cancer and elevated PSA § Tracers that image bone § F-18 Na. F (sodium fluoride) § Used to detect bone metastases

PET/CT in Prostate Cancer: NCCN Guidelines § C-11 choline: Radioactive diagnostic agent for PET imaging of patients with suspected prostate cancer recurrence and noninformative bone scintigraphy, CT or MRI. In these patients, C-11 choline-PET/CT imaging may help identify potential sites of prostate cancer recurrence for subsequent histologic confirmation § F-18 Na. F: Newer technology using F-18 Na. F for PET scanning can be used as a diagnostic staging study; appears to have greater sensitivity than Tc-99 m bone scan for assessing bone metastasis § F-18 FDG: In certain clinical settings, the use of F-18 FDG may provide useful information; F-18 FDG-PET/CT should not be used routinely since data on its utility in prostate cancer is limited § F-18 fluciclovine: Indicated for PET imaging with suspected prostate cancer recurrence based on elevated blood PSA levels following prior treatment. (NOTE: FDA approved fluciclovine May 2016; it is not yet included in the NCCN guidelines. ) www. NCCN. org NCCN Guidelines Prostate Cancer, Version 1. 2016 accessed 3/8/16

Cancer Guidelines: Overview for Prostate Cancer Evaluation The NCCN guidelines have specific recommendations when using PET/CT imaging in prostate cancer: (1) primary disease, (2) biochemical recurrence and (3) advanced disease. Below is a sample of information taken from these guidelines. Please consult the NCCN website for their complete set of guidelines. www. NCCN. org/prostate cancer guidelines accessed 3/8/16 lete p m co ult s n - co bsite y l on we e l N p am t NCC x e For eline a guid

PET/CT in Prostate Cancer: Benefits of C-11 Choline Imaging § CMS may reimburse the use of C-11 choline-PET/CT in biochemical failure (i. e. , relapse of prostate cancer with inconclusive bone scan, CT and/or MRI) § Contact your local MAC for your area’s coverage decision § Established tracer to identify progression to support more appropriate treatment options § Shows soft tissue and osseous metastatic disease § Availability varies due to half-life of 20 minutes Ceci F, et al. EJNMMI 2014; 41(12): 2222 -2231

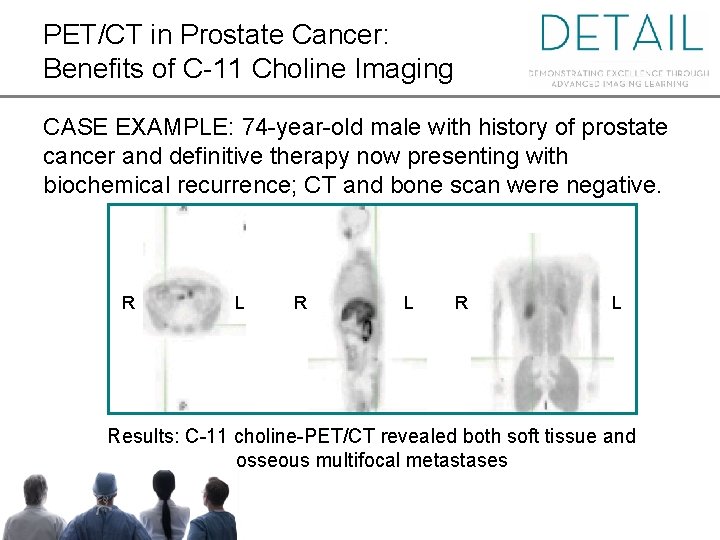
PET/CT in Prostate Cancer: Benefits of C-11 Choline Imaging CASE EXAMPLE: 74 -year-old male with history of prostate cancer and definitive therapy now presenting with biochemical recurrence; CT and bone scan were negative. R L R L Results: C-11 choline-PET/CT revealed both soft tissue and osseous multifocal metastases

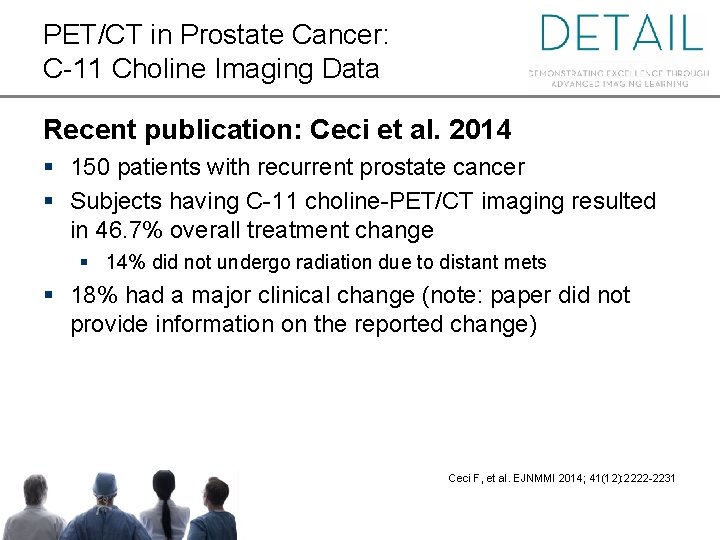
PET/CT in Prostate Cancer: C-11 Choline Imaging Data Recent publication: Ceci et al. 2014 § 150 patients with recurrent prostate cancer § Subjects having C-11 choline-PET/CT imaging resulted in 46. 7% overall treatment change § 14% did not undergo radiation due to distant mets § 18% had a major clinical change (note: paper did not provide information on the reported change) Ceci F, et al. EJNMMI 2014; 41(12): 2222 -2231

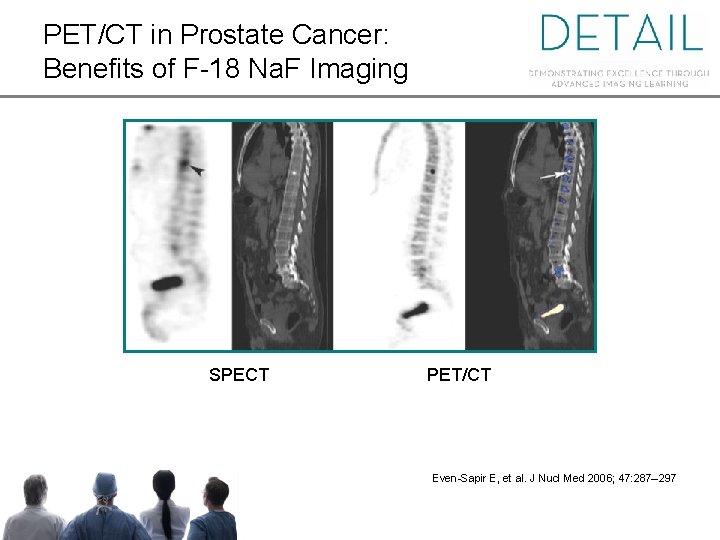
PET/CT in Prostate Cancer: Benefits of F-18 Na. F Imaging SPECT PET/CT Even-Sapir E, et al. J Nucl Med 2006; 47: 287– 297

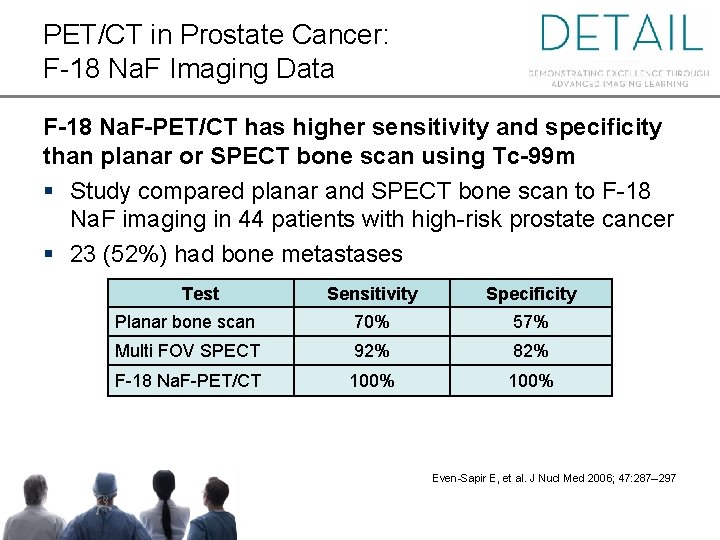
PET/CT in Prostate Cancer: F-18 Na. F Imaging Data F-18 Na. F-PET/CT has higher sensitivity and specificity than planar or SPECT bone scan using Tc-99 m § Study compared planar and SPECT bone scan to F-18 Na. F imaging in 44 patients with high-risk prostate cancer § 23 (52%) had bone metastases Test Sensitivity Specificity Planar bone scan 70% 57% Multi FOV SPECT 92% 82% F-18 Na. F-PET/CT 100% Even-Sapir E, et al. J Nucl Med 2006; 47: 287– 297

PET/CT in Prostate Cancer: F-18 Na. F and NOPR § The National Oncologic PET Registry (NOPR) is a mechanism for CMS Coverage § A registry for F-18 Na. F-PET to identify bone metastasis similar to that now in place for F-18 FDG-PET § Launched February 7, 2011 § >30, 000 scans with complete data submitted to date Hillner B, et al. J Nucl Med 2014; 55: 574 -581

Impact of F-18 Na. F on Prostate Cancer Management F-18 Na. F-PET changed intended management in 77% of cases § Initial no treatment decision revised to treatment in 77% § Switch from initial staging [IS] to primary osseous metastization [POM] § Therapy management changed in 44% to 52% § Consideration for coverage request for F-18 Na. F still under evaluation by CMS Hillner B, et al. J Nucl Med 2014; 55: 574 -581

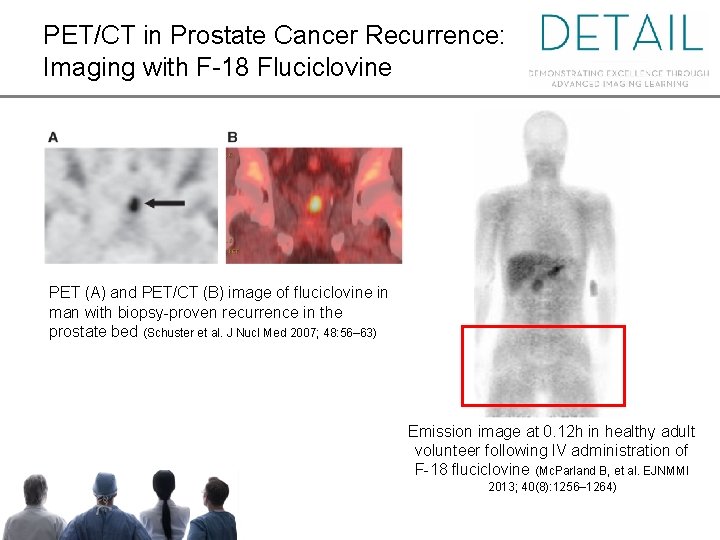
PET/CT in Prostate Cancer Recurrence: Imaging with F-18 Fluciclovine PET (A) and PET/CT (B) image of fluciclovine in man with biopsy-proven recurrence in the prostate bed (Schuster et al. J Nucl Med 2007; 48: 56– 63) Emission image at 0. 12 h in healthy adult volunteer following IV administration of F-18 fluciclovine (Mc. Parland B, et al. EJNMMI 2013; 40(8): 1256– 1264)

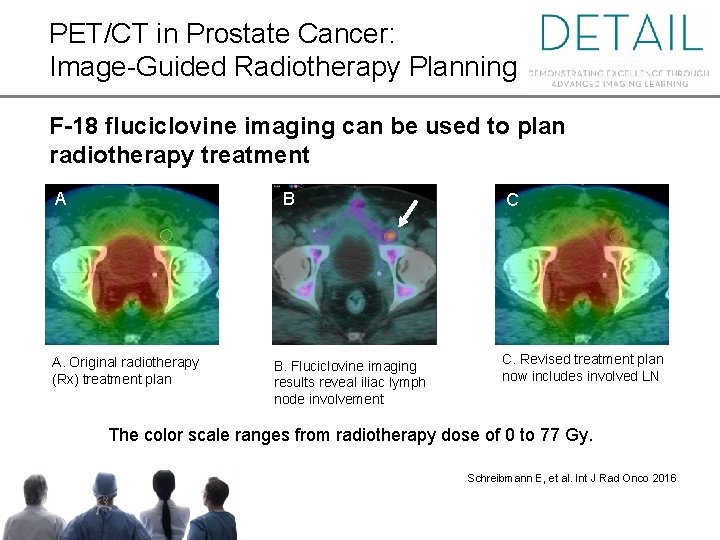
PET/CT in Prostate Cancer: Image-Guided Radiotherapy Planning F-18 fluciclovine imaging can be used to plan radiotherapy treatment A B A. Original radiotherapy (Rx) treatment plan B. Fluciclovine imaging results reveal iliac lymph node involvement C C. Revised treatment plan now includes involved LN The color scale ranges from radiotherapy dose of 0 to 77 Gy. Schreibmann E, et al. Int J Rad Onco 2016

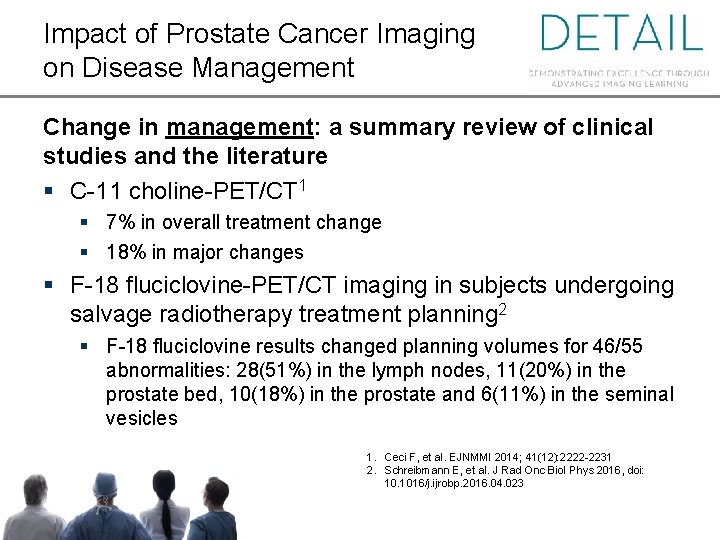
Impact of Prostate Cancer Imaging on Disease Management Change in management: a summary review of clinical studies and the literature § C-11 choline-PET/CT 1 § 7% in overall treatment change § 18% in major changes § F-18 fluciclovine-PET/CT imaging in subjects undergoing salvage radiotherapy treatment planning 2 § F-18 fluciclovine results changed planning volumes for 46/55 abnormalities: 28(51%) in the lymph nodes, 11(20%) in the prostate bed, 10(18%) in the prostate and 6(11%) in the seminal vesicles 1. Ceci F, et al. EJNMMI 2014; 41(12): 2222 -2231 2. Schreibmann E, et al. J Rad Onc Biol Phys 2016, doi: 10. 1016/j. ijrobp. 2016. 04. 023

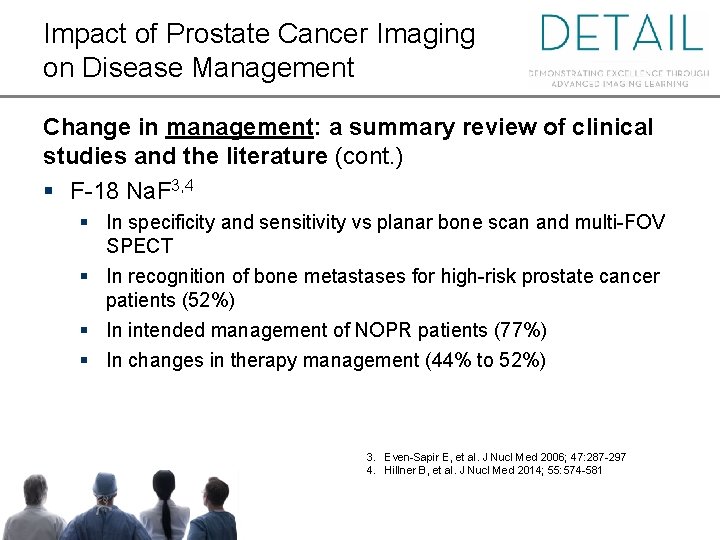
Impact of Prostate Cancer Imaging on Disease Management Change in management: a summary review of clinical studies and the literature (cont. ) § F-18 Na. F 3, 4 § In specificity and sensitivity vs planar bone scan and multi-FOV SPECT § In recognition of bone metastases for high-risk prostate cancer patients (52%) § In intended management of NOPR patients (77%) § In changes in therapy management (44% to 52%) 3. Even-Sapir E, et al. J Nucl Med 2006; 47: 287 -297 4. Hillner B, et al. J Nucl Med 2014; 55: 574 -581

Key Messages § PET/CT is a powerful non-invasive diagnostic tool § Effective for: Detecting metastatic disease, restaging, identifying biochemical relapse post-radical therapy, monitoring treatment and primary staging generally limited to only high-risk disease § F-18 Na. F imaging is an important tool for evaluating bone mets § Indications for using PET/CT in prostate cancer patients § Negative CT/MRI/SPECT bone scan and rising PSA § Radiation therapy planning § Staging of aggressive tumor types § F-18 FDG and F-18 fluciclovine show utility in detecting local and/or regional and distant recurrence

References § www. NCCN. org Prostate Cancer Guidelines v. 1. 2016 accessed 3/8/16 § Ceci F, Herrmann K, Castellucci P, et al. Impact of 11 C-choline PET/CT on clinical decision making in recurrent prostate cancer: results from a retrospective two-centre trial. EJNMMI Dec 2014; 41(12): 2222 -2231 § Even-Sapir, E. Imaging of Malignant Bone Involvement by morphologic, scintigraphic, and hybrid modalities. J Nuc Med 2005; 46: 1356 -1367 § Even-Sapir E, Metser U, Mishani E, et al. The detection of bone metastases in patients with high-risk prostate cancer: 99 m. Tc-MDP planar bone scintigraphy, single- and multi-Field-of-View SPECT, 18 F-fluoride PET, and 18 F-fluoride PET/CT. J Nucl Med 2006; 47: 287– 297

References § Frederick DG, Fahey FH, Packard AB, et al. Skeletal PET with 18 F-fluoride: Applying new technology to an old tracer. J Nucl Med 2008; 49: 68– 78 § Hillner B, Siegel B, Hanna L, et al. Impact of 18 F-Fluoride PET in patients with known prostate cancer: Initial results from the National Oncologic PET Registry. J Nucl Med 2014; 55: 574 -581 § Jadvar H. Molecular Imaging of Prostate Cancer with PET. J Nucl Med 2013; 54(10): 1685 -1688 § Mc. Parland B, Wall A, Johansson S, and Sørensen J. The clinical safety, biodistribution and internal radiation dosimetry of [18 F]fluciclovine in healthy adult volunteers. EJNMMI 2013; 40(8): 1256– 1264 § Segall G, Delbeke D, Stabin M, et al. SNMMI Practice Guidelines for Sodium 18 F-Fluoride PET/CT Bone Scans 1. 1. JNMMI 2010; 51(11): 18131820

References § Schirrmeister H, Guhlmann A, Kotzerke J, et al. Early detection and accurate description of extent of metastatic bone disease in breast cancer with fluoride ion and positron emission tomography. J Clin Oncol 1999; 17(8): 2381 -2389 a § Schuster D, Votaw J, Nieh P, et al. Initial experience with the radiotracer anti -1 -amino-3 -18 F-fluorocyclobutane-1 -carboxylic acid with PET/CT in prostate carcinoma. J Nucl Med 2007; 48: 56– 63

Important Safety Information § Image interpretation errors can occur with PET imaging. A negative image does not rule out recurrent prostate cancer and a positive image does not confirm its presence. Clinical correlation, which may include histopathological evaluation, is recommended. § The performance of F-18 fluciclovine and C-11 choline seem to be affected by PSA levels. For F-18 fluciclovine, uptake may occur with other cancers and benign prostatic hypertrophy in primary prostate cancer. § Hypersensitivity reactions, including anaphylaxis, may occur in patients who receive PET radiopharmaceuticals. Emergency resuscitation equipment and personnel should be immediately available. § PET/CT imaging contributes to a patient’s overall long-term cumulative radiation exposure, which is associated with an increased risk of cancer. Safe handling practices should be used to minimize radiation exposure to the patient and healthcare providers. § Adverse reactions, although uncommon, may occur when using PET radiopharmaceuticals. Always refer to the package insert prior to use.