Using JMP Scripts to Automate Generation of Graphs











- Slides: 32

Using JMP® Scripts to Automate Generation of Graphs and Analysis for Bio. Pharmaceutical Manufacturing Studies Jessica A. Behrle, Associate Director Yinglei Li, Senior Statistician Quantitative Sciences, Janssen R&D September 21, 2016 Melinda, Tree of Life Melinda’s artwork reflects her journey living with HIV.

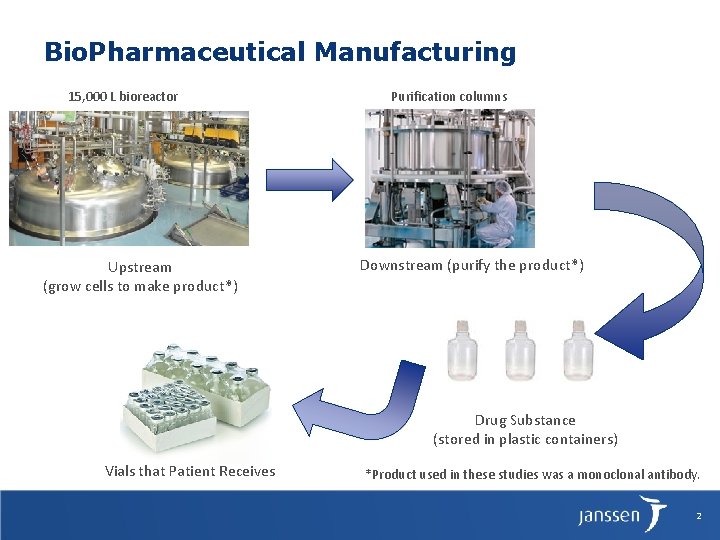
Bio. Pharmaceutical Manufacturing 15, 000 L bioreactor Upstream (grow cells to make product*) Purification columns Downstream (purify the product*) Drug Substance (stored in plastic containers) Vials that Patient Receives *Product used in these studies was a monoclonal antibody. 2

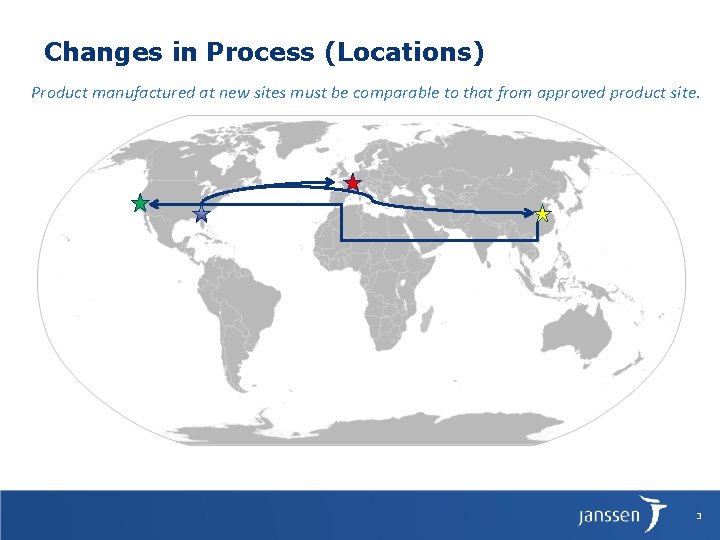
Changes in Process (Locations) Product manufactured at new sites must be comparable to that from approved product site. 3

Other Changes Delivery Method(s) Intravenous (IV) product Injectable product Dose Downstream Processing Changes Purification Columns 100 mg/400 mg vials 4

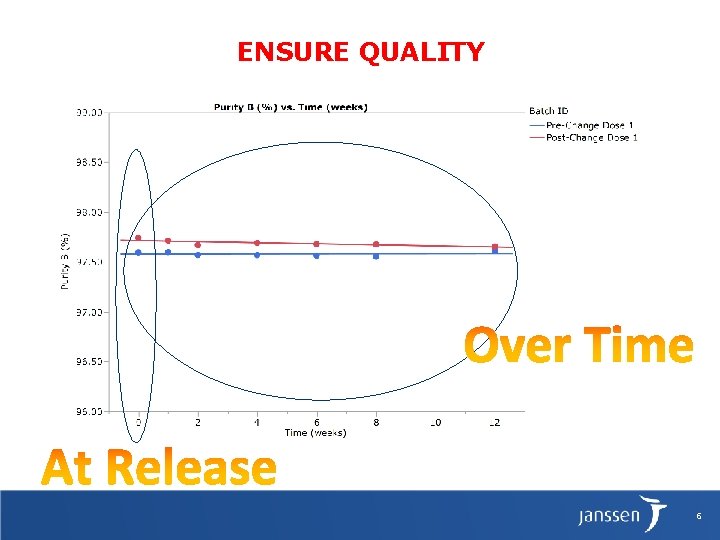
Why degradation studies? § Regulatory Guidance ICH Q 5 E (www. ich. org) COMPARABILITY OF BIOTECHNOLOGICAL/BIOLOGICAL PRODUCTS SUBJECT TO CHANGES IN THEIR MANUFACTURING PROCESS – Manufacturer must prove that manufacturing process changes will not adversely impact product quality, safety and efficacy: § At release (time 0) § During storage: Stability data, including those generated from accelerated or stress conditions, is used to evaluate any differences in the degradation pathways of the pre-change and post-change product. – Involves significant analytical testing to evaluate different structural features. (Protein structure is frequently sensitive to a variety of process changes. ) 5

ENSURE QUALITY 6

Stress Condition: Heat 5˚C = 41˚F A cold day 25˚C = 77˚F A warm day 40˚C = 104˚F A hot day https: //www. mathsisfun. com/measure/thermometer. html 7

Why do we need a slope comparison? • Comparing the slopes on stability is a Quantitative assessment of the data • Are the pre and post degrading or changing over time at the same rate? • Minimize subjectivity • Assuming linear slopes & normal data • Science plays a role in interpreting the statistical differences. • P-values are a guide but also need to see degradation. 8

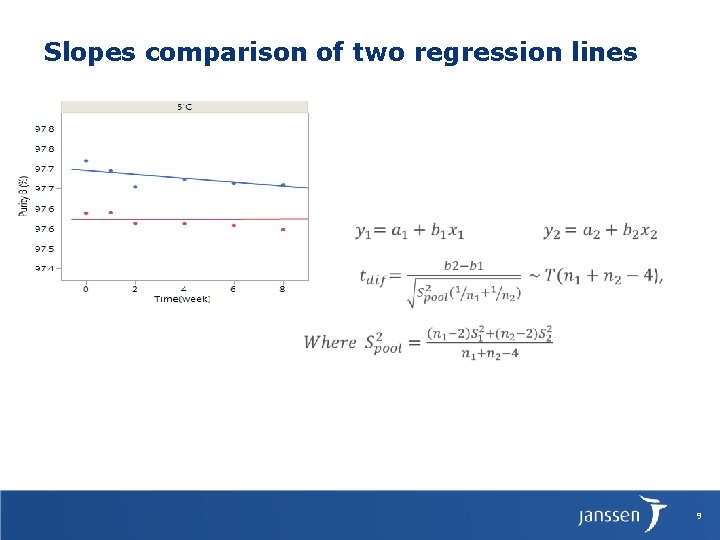
Slopes comparison of two regression lines 9

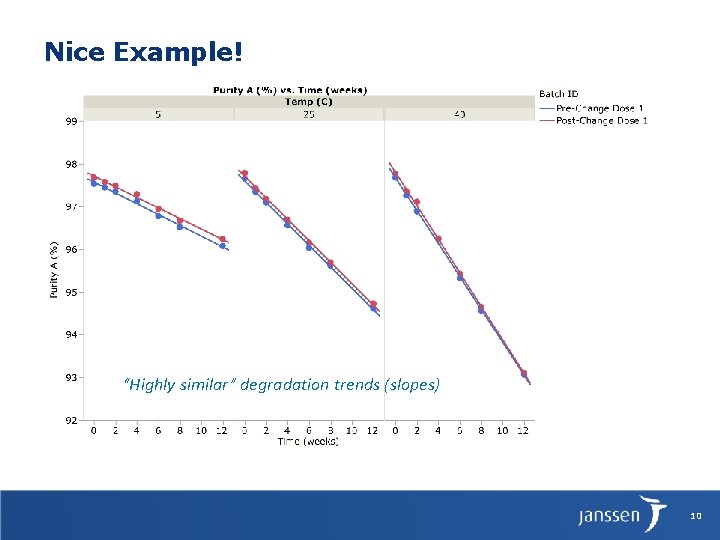
Nice Example! “Highly similar” degradation trends (slopes) 10

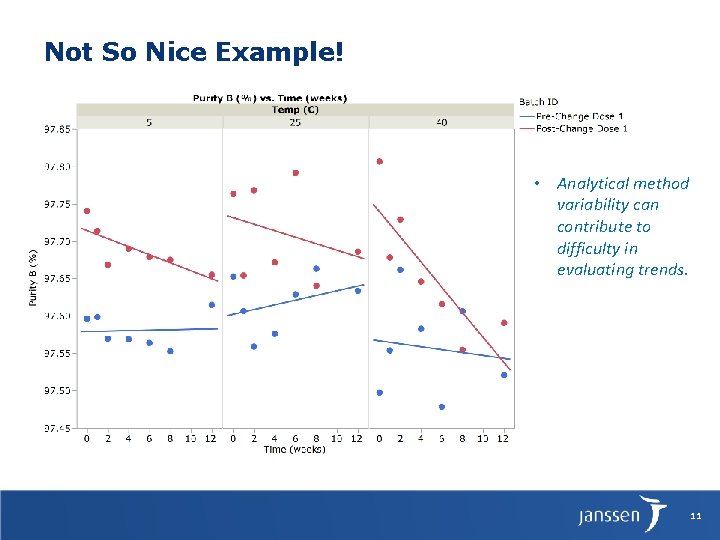
Not So Nice Example! • Analytical method variability can contribute to difficulty in evaluating trends. 11

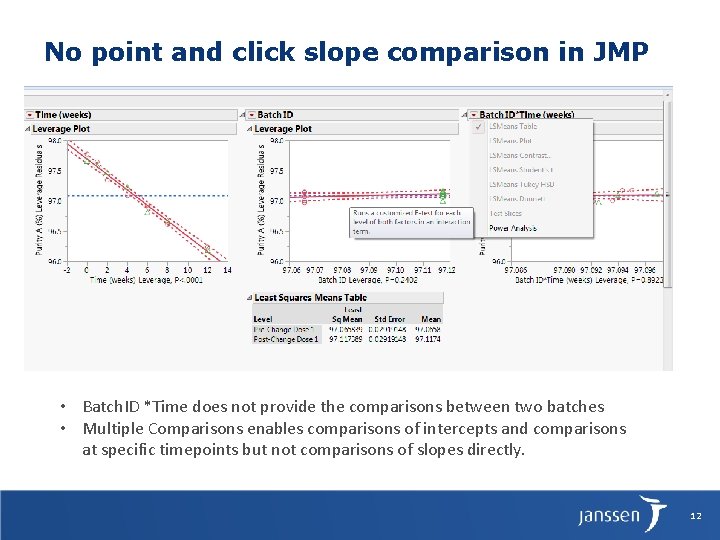
No point and click slope comparison in JMP • Batch. ID *Time does not provide the comparisons between two batches • Multiple Comparisons enables comparisons of intercepts and comparisons at specific timepoints but not comparisons of slopes directly. 12

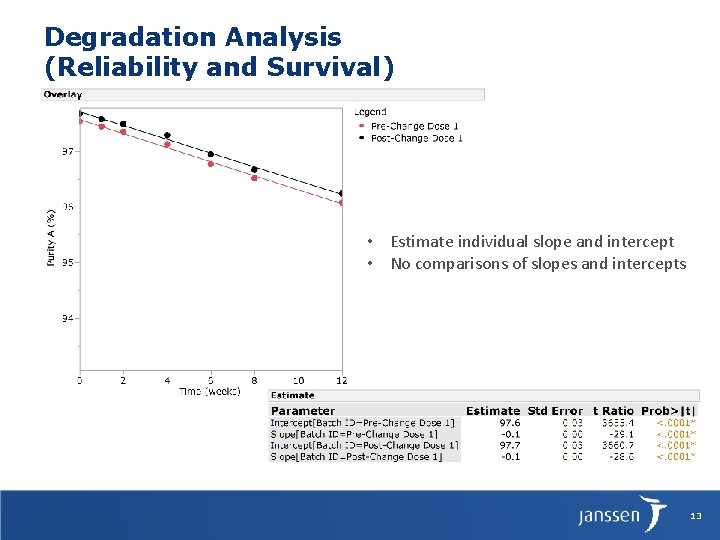
Degradation Analysis (Reliability and Survival) • Estimate individual slope and intercept • No comparisons of slopes and intercepts 13

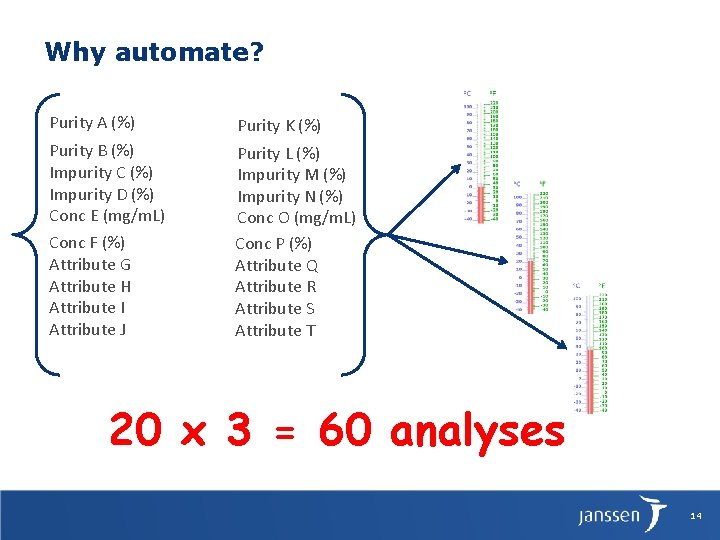
Why automate? Purity A (%) Purity K (%) Purity B (%) Impurity C (%) Impurity D (%) Conc E (mg/m. L) Purity L (%) Impurity M (%) Impurity N (%) Conc O (mg/m. L) Conc P (%) Attribute Q Attribute R Attribute S Attribute T Conc F (%) Attribute G Attribute H Attribute I Attribute J 20 x 3 = 60 analyses 14

Even more reasons for Automation • • Scripts to perform the slope comparison for all attributes Generate all the Graphs Consistent study design for these studies Enable scientists to analyze 15

Objective § Graphically and statistically evaluate the trends and differences in the trends between the batches for all storage conditions and attributes § Aim to develop automation tool 16

Example & Demonstration

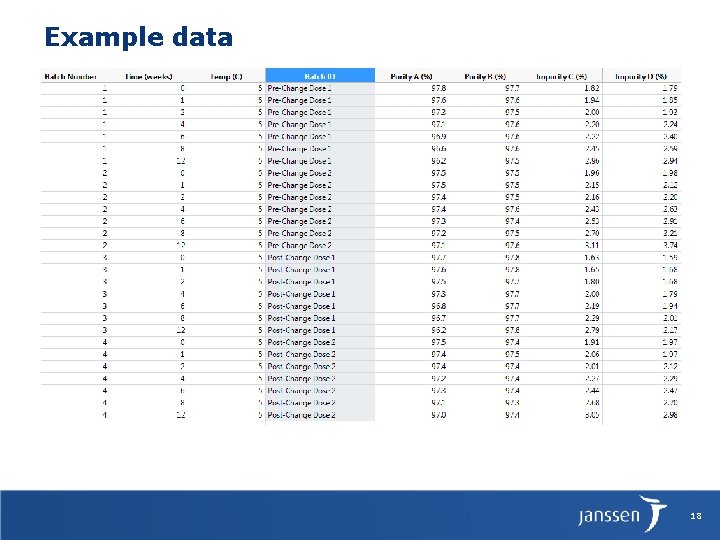
Example data 18

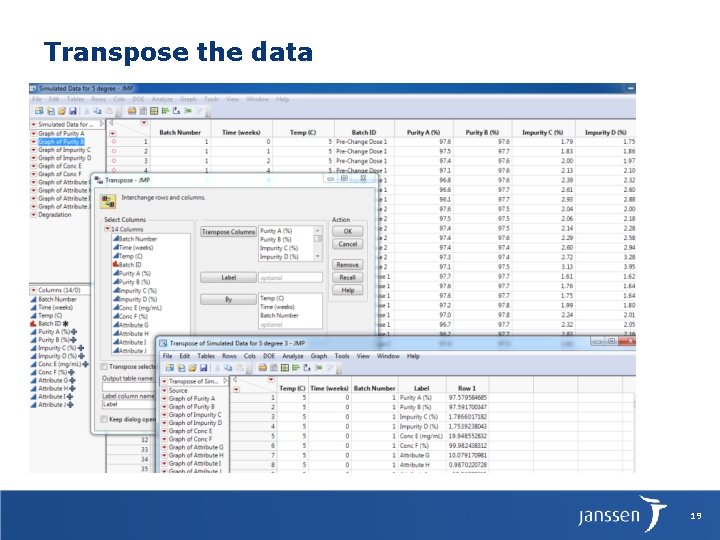
Transpose the data Subset the data by pairs of pre-changes vs post-change batches 19

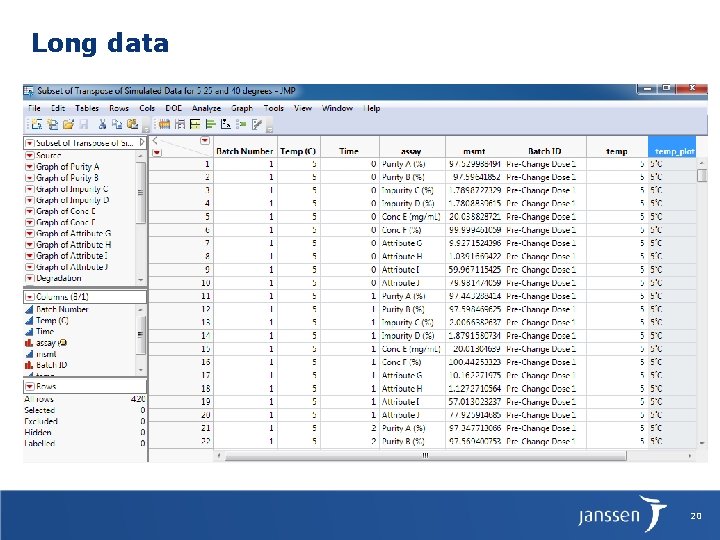
Long data 20

Graphs code § Basic graph by attributes for a loop 21

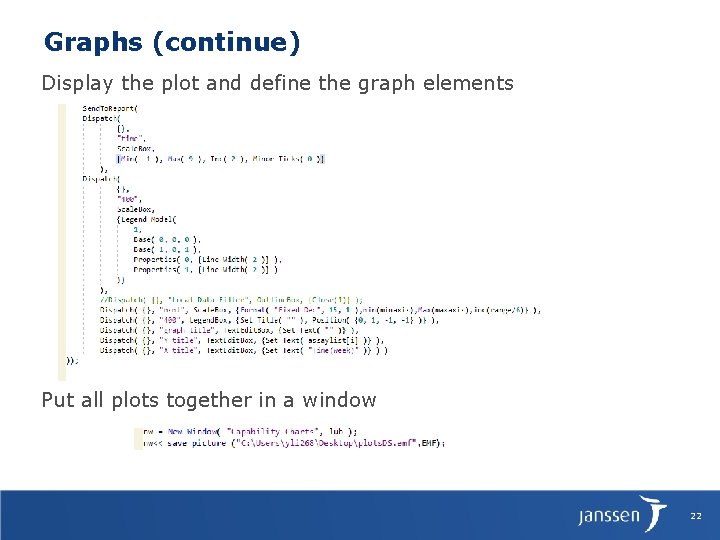
Graphs (continue) Display the plot and define the graph elements Put all plots together in a window 22

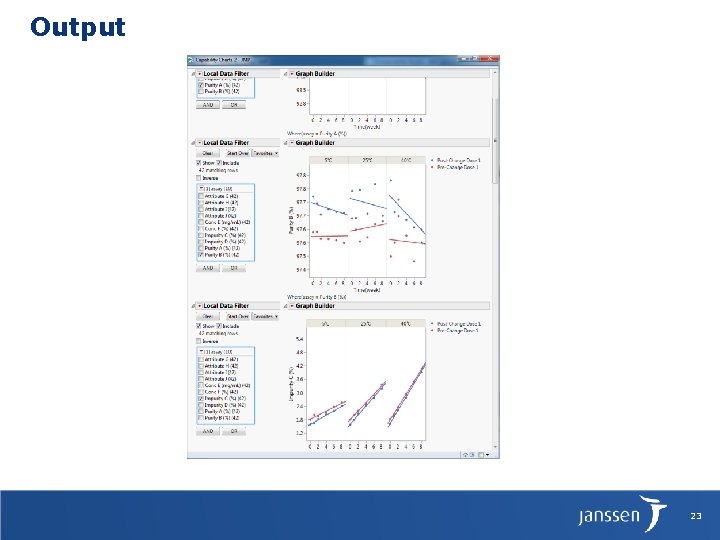
Output 23

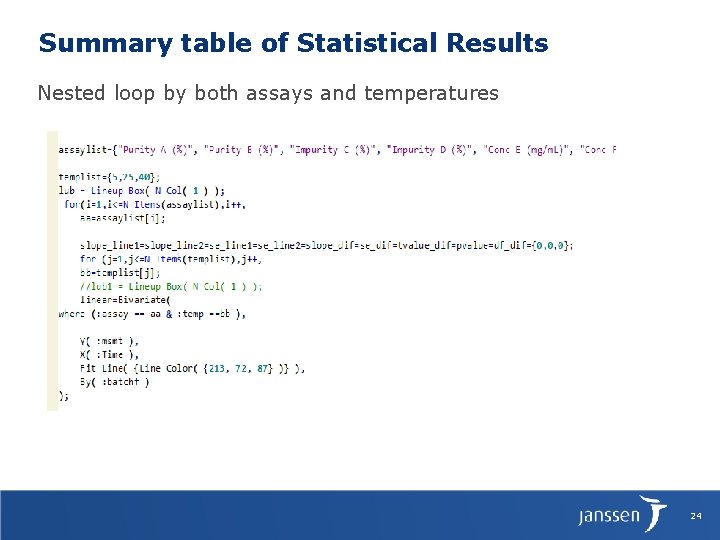
Summary table of Statistical Results Nested loop by both assays and temperatures 24

Tables (Continue) 25

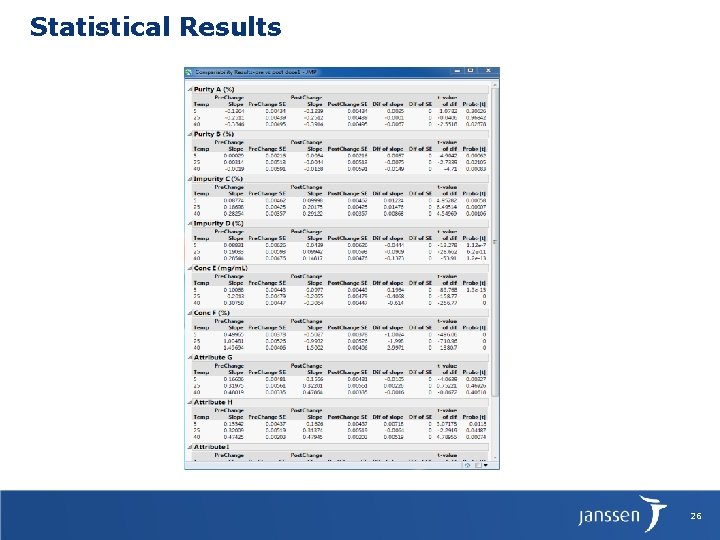
Statistical Results 26

More code tips from my scripts § Append add a display box for each variable in the analysis to the bottom of an existing display § Send To Report and Dispatch commands are useful to customize the appearance of a plot § Table box is to create a table with Number Col Box for a numerical column and string col box for a string column § New Window command displays all graphs in a window 27

Learning Scripting tips § JMP/SAS blog---provides kinds of search question results § Book: Jump into JMP Scripting; Scripting Guide PDF in JMP § View, edit and experiment with scripts created using JMP Commands § Searching the help Index § Troubleshooting 28

Future Considerations § Statistics Considerations – Analyzing all batches together – Adjusting for Multiplicity – Variability (pooled vs unpooled etc. ) used in evaluating the differences – Equivalency testing – CI on differences – Comparisons of intercepts § Scripting Considerations – User defined attributes/temperatures to analyze – Creating one report file of all graphs and tables (i. e. , easy exportation to word to merge into a report) 29

References § Wendy Murphrey; Rosemary Lucas. Jump Into Jmp Scripting. SAS Institute. ISBN 978 -1 -59994 -658 -0. Retrieved 14 December 2012. § SAS Publishing (1 March 2012). Jmp 10 Scripting Guide. SAS Institute. ISBN 978 -1 -61290 -195 -4. Retrieved 13 December 2012. § J. M. Andrade, M. G. Estévez-Pérez. Statistical comparison of the slopes of two regression lines: a tutorial. Anal Chim Acta, 838 (2014), pp. 1– 12 30

Thank you Barry Hogan, Senior Scientist, Janssen R&D Yonghui Wang, Senior Scientist, Janssen R&D Michael Nedved, Associate Director, Janssen R&D Jessica A. Behrle, jbehrle@its. jnj. com Yinglei Li, yli 268@its. jnj. com September 21, 2016 Melinda, Tree of Life Melinda’s artwork reflects her journey living with HIV.
